Figures & data
Figure 1. (A) Chemical structure of bile salts commonly used in bile salts-containing vesicles and (B) an illustration depicting the hydrophilic side and hydrophobic side of a bile salt molecule.
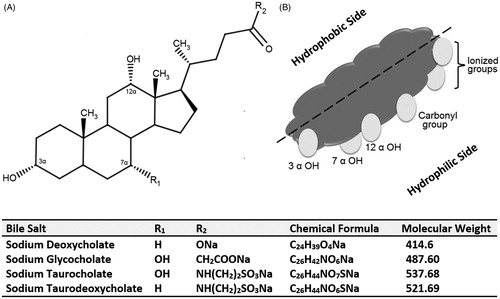
Figure 2. A schematic representation of the behavior of conventional liposome and niosome in comparison to bilosomes in presence of intestinal bile salts. (A) Disruption and lysis of conventional vesicle by intestinal bile salts and (B) The bilosome remains stable in presence of intestinal bile salts (redrawn from Henriksen-Lacey & Perrie, Citation2013).
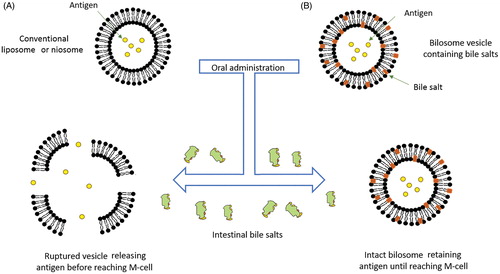
Table 1. Commonly used lipids, surfactants and bile salts for preparing BS-vesicles.
Figure 3. Structural composition of different types of liposomes. (A) Conventional liposome, (B) Bile salts-containing liposome and (C) Bile salts coated liposome.
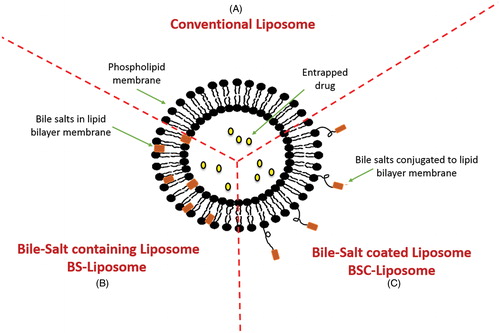
Figure 4. Schematic representation of the uptake of bile salts liposomes by intestinal epithelium after oral administration. (A) Uptake of the vesicles by M-cells in the Payer’s patch, (B) Transport of the vesicles through the paracellular pathway and (C) Transcellular uptake of vesicles through the enterocytes.
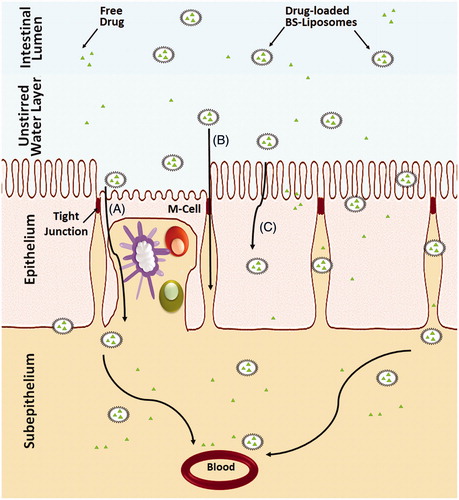
Figure 5. Structural composition of different types of bilosomes. (A) Conventional bilosome, (B) Glucomannan bilosome and (C) Cholera toxin B-bilosome.
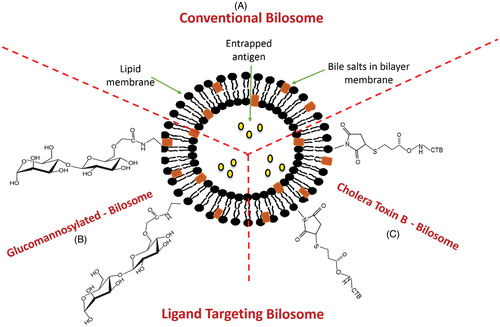
Figure 6. Schematic representation of the uptake of bilosomes containing antigen through the intestinal epithelium after oral administration. (A) Transcellular uptake of bilosomes through the enterocytes, (B) Transport of the bilosomes through paracellular pathway and (C) Uptake of bilosomes by M-cells and dendritic cells.
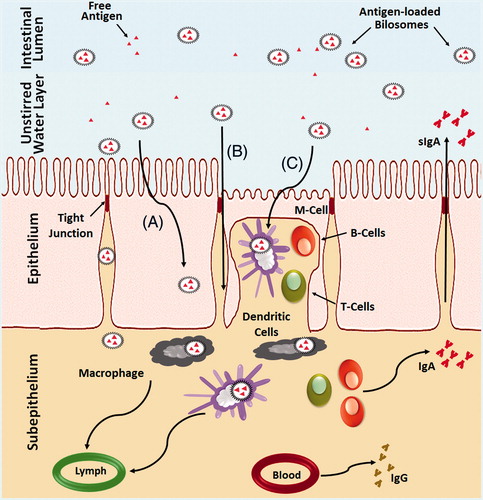
Figure 7. Representation of different methods adopted for preparation of bile salts-containing vesicles. (A) Reverse phase evaporation method, (B) Thin film hydration method and (C) Hot homogenization method.
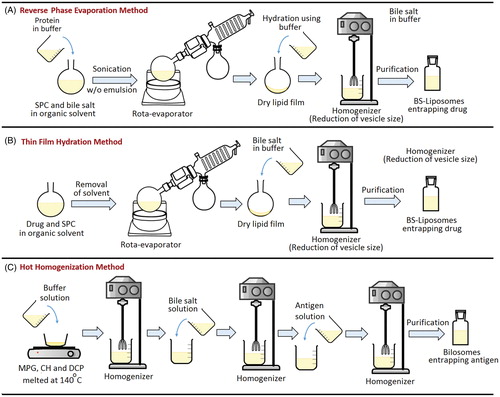
Table 2. Formulation details, preparation methods, and vesicles characteristics of bile salt-containing liposomes (BS-liposomes).
Table 3. Formulation details, preparation methods and vesicles characteristics of bilosomes.
Table 4. Formulation details, preparation methods and vesicles characteristics of modified bilosomes.
Table 5. Major methods for BS-vesicles characterizations.
Figure 8. In vivo performance of orally administrated bile salts-containing liposomes in comparison to conventional liposomes. (A) Fenofibrate-loaded bile salts-containing liposomes (Chen et al., Citation2009) and (B) Recombinant insulin-loaded bile salts-containing liposomes (Niu et al., Citation2014).
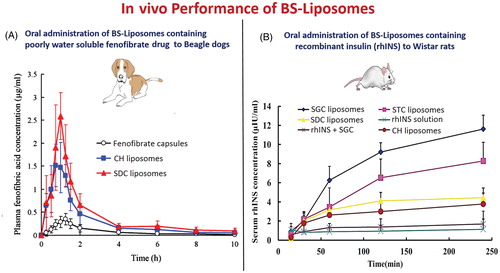
Table 6. In vivo performances of BS-liposomes in different animal models.
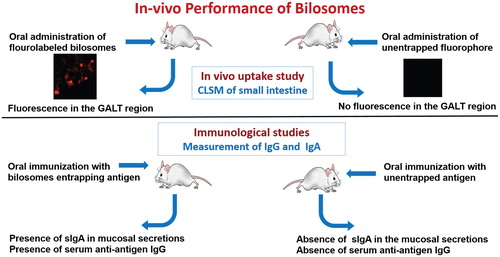
Figure 9. In vivo performance of orally administrated bilosomes. (A) Confocal laser scanning microscopy (CLSM) of the intestine (Shukla et al., Citation2011) and (B) Immunological study and measurement of specific IgA and IgG.