Figures & data
Figure 1. Purification of IgY antibodies and the function assay. (A) SDS–PAGE analysis of purified IgY against toxin A. (B) The titer of chicken yolk antibodies against ToxA–C in eggs. (C) The injection ToxA and IgY antibodies of intestinal loops: (1) 100 µg/ml ToxA, (2) 50 µg/ml ToxA, (3) normal saline, (4) 100 µg/ml ToxA and IgY antibodies and (5) 50 µg/ml ToxA and IgY antibodies.
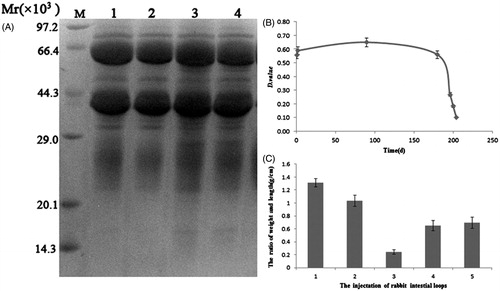
Figure 2. Stability of IgY in the loaded microbeads. (A) pH stability of IgY in the loaded microbeads. The IgY-loaded microbeads were suspended in PBS buffer with different pH (2–11) value for 24 h, the buffer was aliquoted for ELISA assay to determine the binding activities of the IgY antibodies at 1, 12 and 24 h. IgY antibodies were stable between pH 4–10. (B) Temperature stability of IgY in the loaded microbeads. IgY-loaded microbeads were put in the incubators of different temperatures for 1, 12 and 24 h, then the IgY antibodies were dissolved in PBS (pH 7.4) and the antigen-binding activities of the IgY antibodies were assayed by ELISA. IgY antibodies were stable <60°C. (C) Long-term storage stability of IgY at room temperature. IgY-loaded microbeads were put in the incubator at 25°C without light for 1–6 months, then the IgY antibodies were dissolved in PBS (pH 7.4), antigen-binding activities of IgY antibodies were assayed by EIA. IgY antibodies were stable for at least 6 months if stored at room temperature.
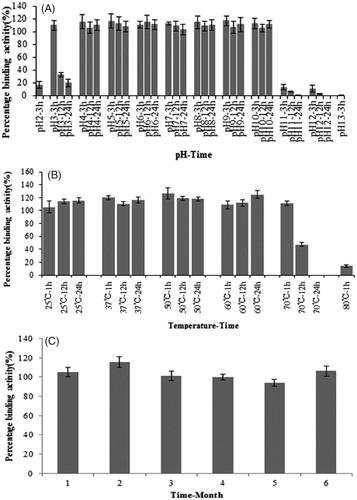
Figure 3. Eudragit S100 coated IgY microbeads. (A) The unloaded beads. (B) IgY-loaded beads. The loading efficiency was 21%. (C) Coated microbeads. The coating rate was 35%. (D) The effect of coating process on the IgY biological activity in the microbeads. (1) Drug-loaded microbeads, (2) coated pellets of Eudragit S100 (aqueous dispersion solvent) and (3) coated pellets of Eudragit S100 (organic solvent).
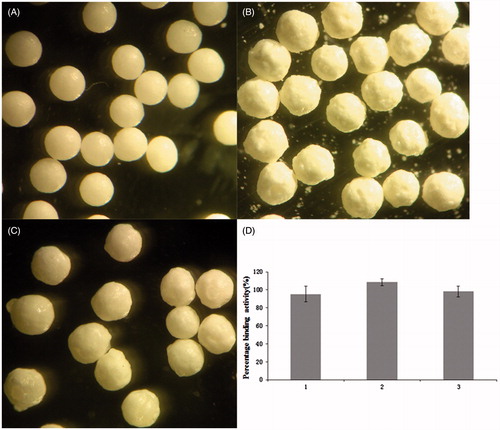
Figure 4. In vitro release of ToxA–C antigen binding-specific IgY from the microbeads. (A) In vitro release The IgY-loaded microbeads. (B) In vitro IgY release from the microbeads in exterosoluble capsules. The IgY-loaded microbeads or the capsules were suspended in artificial gastric fluid for 2 h, artificial intestinal fluid for 6 h and artificial colonic fluid for 4 h, the fluids were sampled every 30 min to detected IgY concentrations using EIA and BCA methods.
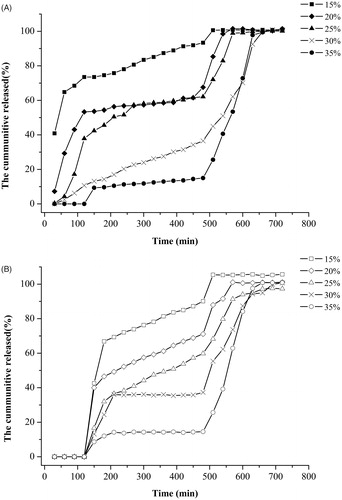
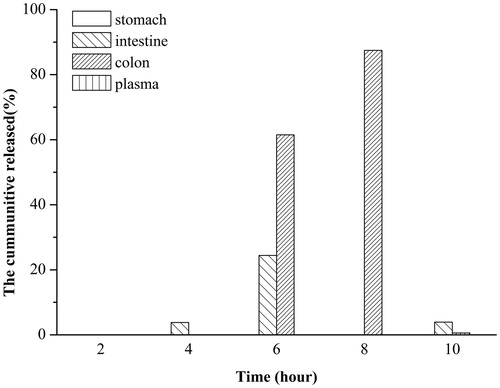
Figure 5. In vivo IgY release in the rat digestion system. The rats were intragastrically administrated with same amount of microbeads containing 10 mg IgY against the ToxA of C. difficile. Stomach, intestine, caecum and colon samples were collected every 2 h. Blood sample from each rat was also collected. After visual examination, 10 ml of PBS (pH 7.4) was added to each sample and homogenated. After supersonication and centrifugation at 10 000 rpm for 30 min at 4°C, the supernatant from each sample was recovered for the assay of IgY concentration in the organs using ELISA method.