Figures & data
Figure 1. Results obtained from various studies involved in the preparation of polymeric nanoparticles. (a) Effect of different surfactants/concentration on particle size and PDI: Tween 20 at a concentration of 2% gave the least particle size. (b) Effect of polymer concentration: particle size was decreased with an increase in polymer concentration. (c) Size and entrapment efficiency of optimized, drug-loaded nanoparticles. (d) Nanoparticles exposed to three freeze thaw cycles at 30 and −20 °C and no significant change in size and PDI was observed. (e) Intensity-based size distribution report of co-encapsulated nanoparticles. (f) TEM image of prepared nanoparticles. Values are expressed as mean ± SD, n = 3.
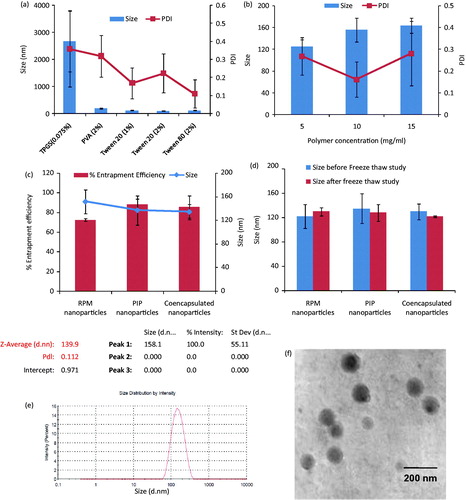
Figure 2. In vitro release profiles of RPM and PIP from (a) individually drug-loaded nanoparticles and (b) co-encapsulated drug nanoparticles and (c) free drug solutions. Study was carried out in dialysis membrane at 37 °C, and normal saline: IPA (90:10, %v/v) was used as release medium.
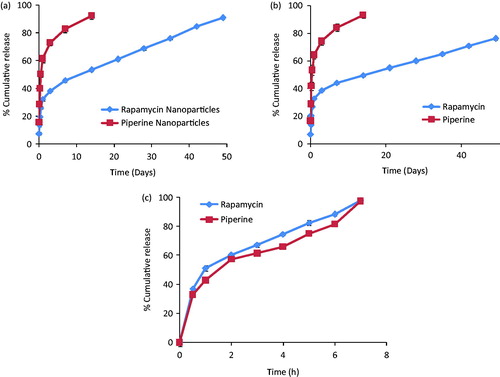
Table 1. Drug release profile and kinetics from individual and co-encapsulated nanoparticles.
Figure 3. Percent permeation of RPM over intestinal barrier using everted gut sac method. Gut sacs were maintained with proper aeration for a period of 90 min. Effect of PIP as a Pgp modulator and potential of nanoparticulate delivery system on intestinal transport of RPM were studied.
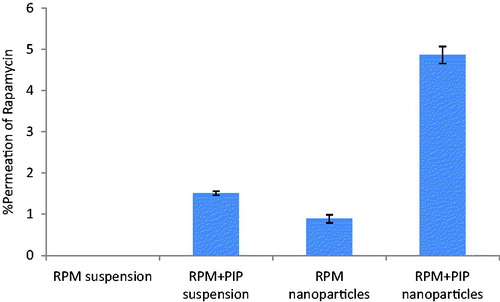
Figure 4. Percent cytotoxicity of different concentrations of rapamycin solution, rapamycin-loaded nanoparticles and blank nanoparticles. Cells were incubated with these formulations for 24 h. Drug-loaded nanoparticles were more efficacious in killing breast cancer cells.
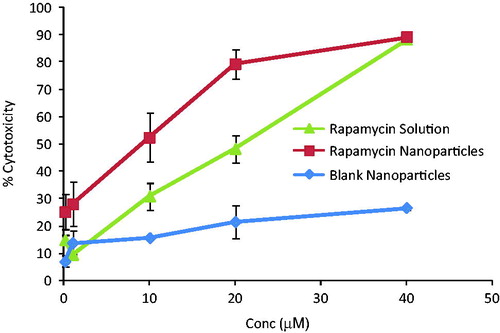
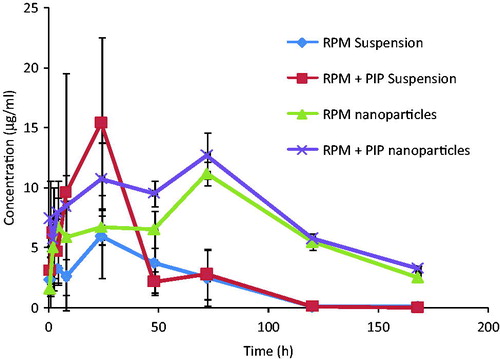
Figure 5. Mean blood concentration–time profiles of rapamycin in female SD rats following oral administration of (a) RPM suspension, (b) RPM-loaded nanoparticles, (c) RPM suspension and PIP suspension and (d) RPM-loaded nanoparticles and PIP-loaded nanoparticles at a single dose of 10 mg/kg for both the drugs (mean ± SD, n = 6).