Figures & data
Table 1. Mean (±S.D.) percentage transmittance of formulation with different Smix at 650 nm, 25 °C (n = 3).
Figure 3. Pseudo-ternary diagram of system with different ratio of surfactant (T80 + S80) to co-surfactant (PEG + Etoh) indicating O/W nanoemulsion region at different Smix ratios. (A) Smix ratio = 1:0; (B) Smix ratio = 1:1; (C) Smix ratio = 1:2; (D) Smix ratio = 1:3; (E) Smix ratio = 2:1.
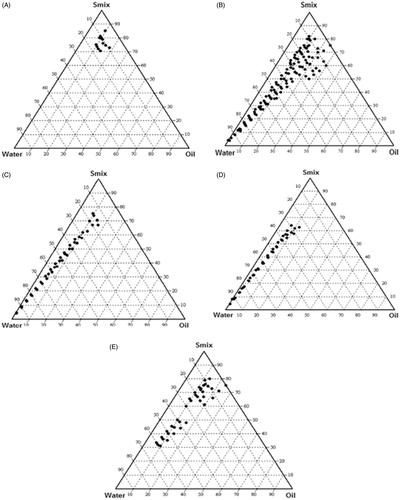
Figure 4. Pseudo-ternary diagram indicating O/W nanoemulsion region at different co- surfactant ratio. (A) PEG/Etoh = 1/1; (B) PEG/Etoh = 2/1; (C) PEG/Etoh = 1/2; (D) PEG/Etoh = 3/1.
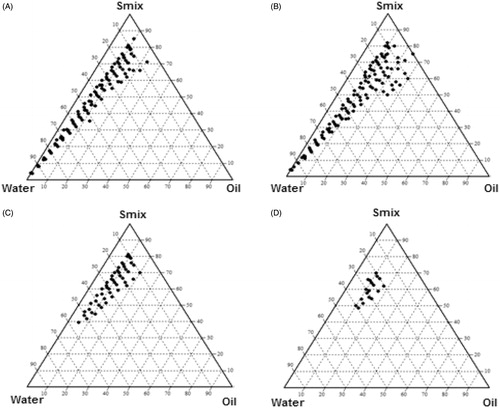
Table 2. Thermodynamic stability test of different formulations selected from phase diagram.
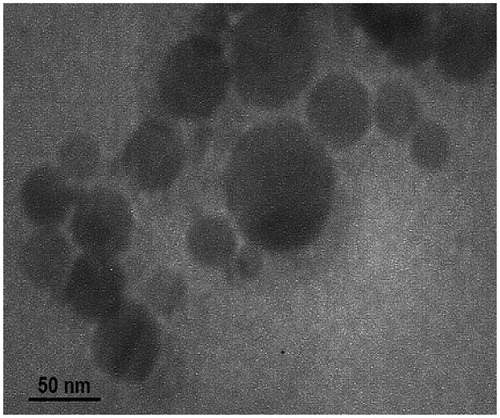
Table 3. Composition, droplet size and polydispersity index of selected nanoemulsion formulations.
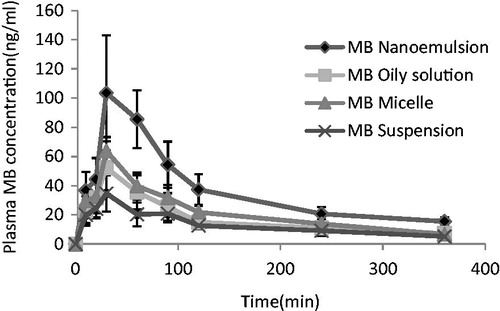
Figure 6. Drug-concentration time profiles of various mebudipine (MB) formulations after oral administration to rats (n = 6, dose = 10 mg/kg).