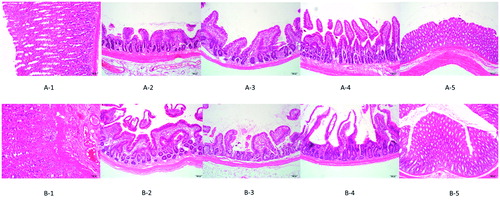
Figures & data
Figure 1. (A) Chemical structure of icariside II. (B). Chemical structure of Solutol®HS15. (C). Chemical structure of Pluronics F127.
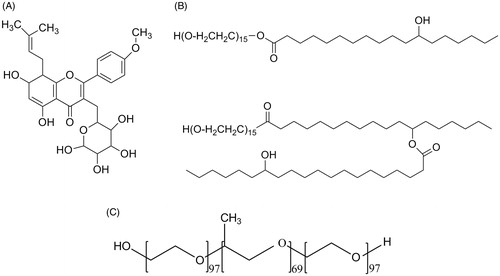
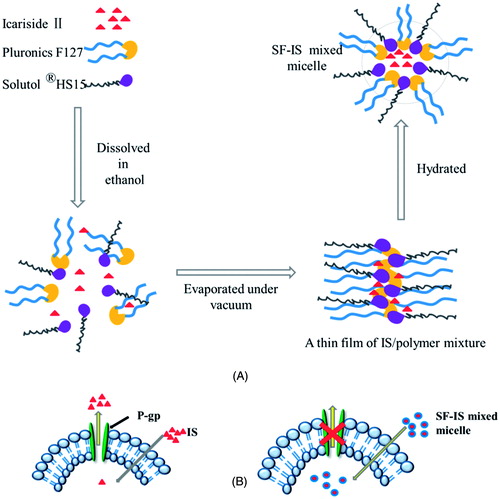
Figure 2. (A) Size distribution of SF mixed micelles as determined by DLS. (B) TEM images of SF-IS mixed micelles.
Table 1. HS15/F127 ratio and characteristics of SF mixed micelles.
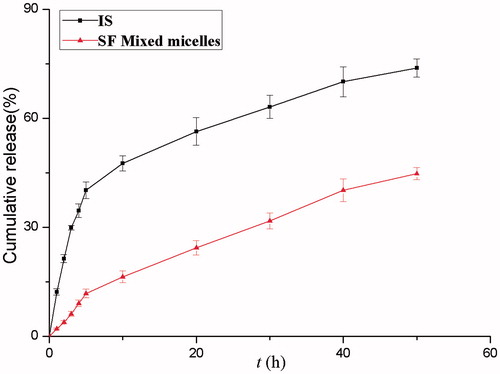
Figure 4. (A) IS. (B) SF (4:1) mixed micelles. Effects of micelles in permeability of IS (20 μM). Absorptive permeability is expressed as Papp (AP-BL) and secretory permeability is expressed as Papp (BL-AP). Date are presented as mean ± SD (n = 3). The asterisk symbol indicates a statistically significant difference. The number of asterisk symbol indicates the level of significance with **p < 0.01. One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
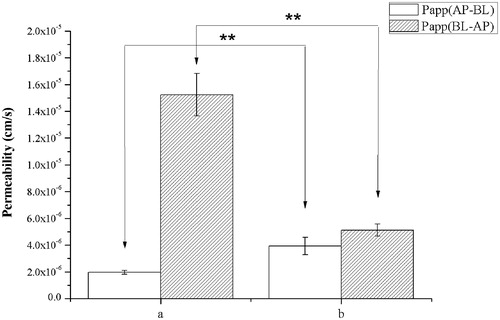
Table 2. Permeability and efflux ratio.
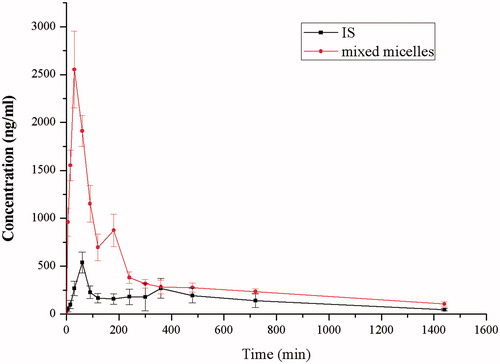
Figure 5. The plasma concentration-time curve of IS in rats after oral administration of IS, SF mixed micelles (100 mg/kg, IS). Data are presented as mean ± SD (n = 6).