Figures & data
Figure 1. Effect of temperature on the activity of immobilized (•) and free (▴) enzyme at pH 6.5. The highest activity is referred to as 100%.
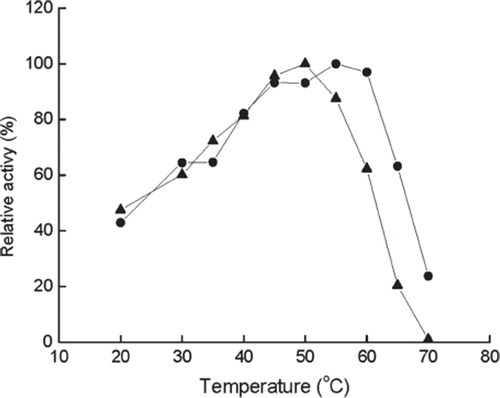
Figure 2. Effect of pH on the activity of immobilized (•) and free (▴) enzymes at 40°C. The highest activity is referred tp as 100%. Buffers (0.05M): pH 2.2–3.4, citrate buffer, pH 4.0–5.4, acetate buffer; pH 6.0–8.0, phosphate buffer.
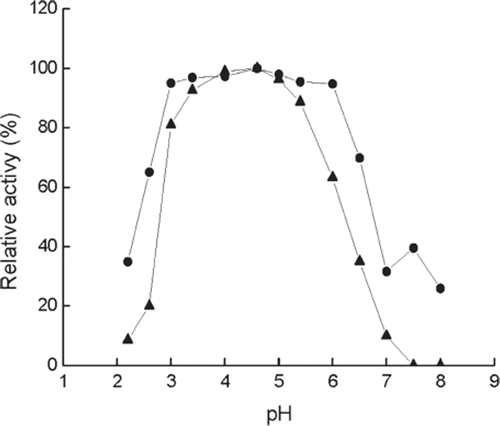
Figure 3. Thermal stability of immobilized (•) and free (▴) enzymes at different temperature and pH 6.5 for 24 h. The highest activity is referred to as 100%.
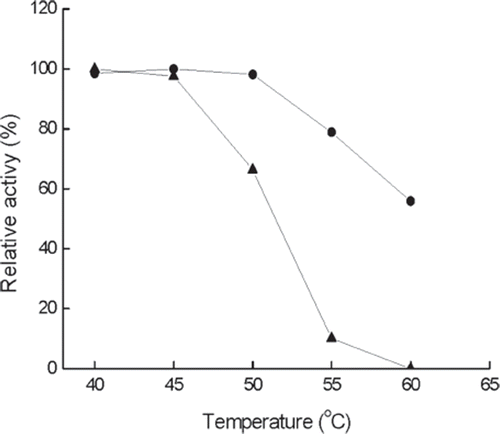
Figure 4. Stability of immobilized (•) and free (▴) enzymes at different pH values and 4°C for 24 h. The highest activity is referred to as 100%. Buffers (0.05 M): pH 2.2–3.4, citrate buffer; pH 6.0–8.0, phosphate buffer.
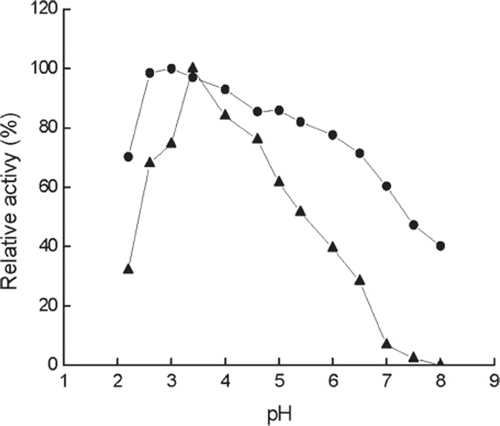
Figure 5. Storage stability of immobilized and free enzymes at 4°C. The highest activity is referred to as 100%. ▴, free enzyme in phosphate buffer (0.05 M, pH 6.5); •, immobilized enzyme in 0.045 M CaCl2 aqueous solution; ♦, immobilized enzyme in 0.135 M CaCl2 aqueous solution; ▾, immobilized enzyme in 0.225 M CaCl2 aqueous solution; ◂, immobilized enzyme in 0.09 M NaCl aqueous solution.
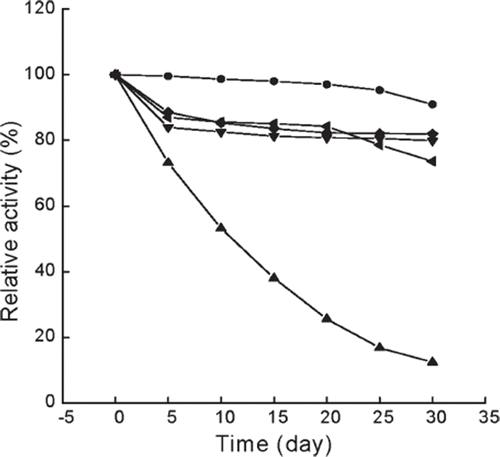
Figure 6. Variations in the relative activities versus operational cycles for the immobilized enzymes prepared or treated with different methods. ▪, normal immobilized method, as control; •, co-immobilized with kaolin; ▴, co-immobilized with diatomite; ▾, crosslinked with glutaradeyde after immobilization; ♦, presence of 0.135 M CaCl2 in reaction solution; ◂, immersion in 0.225 M CaCl2 aqueous solution after each cycle, the activity is determined after the immersion for 0.5h. The intial activity of the control is referred to as 100%.
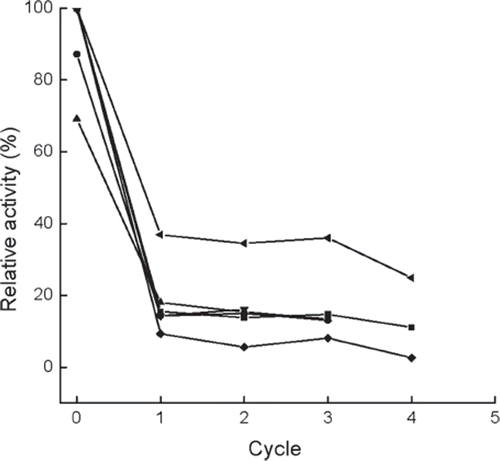
Figure 7. Variations in swelling degrees versus operational cycles for the immobilized enzymes prepared or treated with different methods. ▪, normal immobilized method, as control; •, co-immobilized with kaolin; ▴, co-immobilized with diatomite; ▾, crosslinked with glutaradeyde after immobilization; ♦, presence of 0.135 M CaCl2 in reaction solution; ◂, immersion in 0.225 M CaCl2 aqueous solution after each cycle, swelling degree is detemined afterthe immersion for 0.5h. Swelling degree is defined as the ratio of theweight of 26 beads of the immobilized enzyme with relatively even particle size before the first cycle to that after reaction of the corresponding cycle.
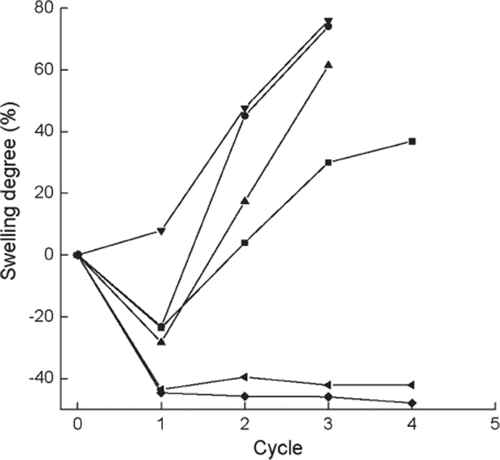
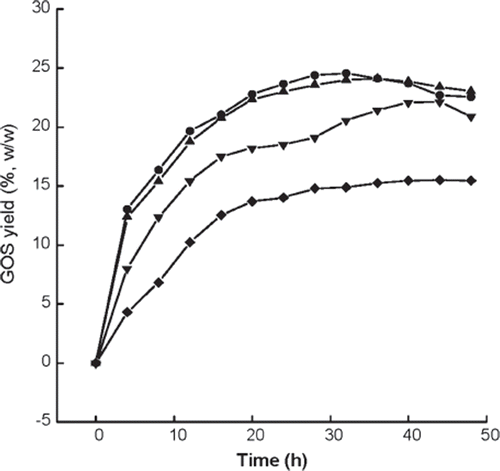
Figure 8. Variations in GOS yields versus operational cycles for the immobilized enzymes prepared or treated with different methods. ▪, normal immobilized method, as control; •, co-immobilized with kaolin; ▴, co-immobilized with diatomite; ▾, crosslinked with glutaradeyde after immobilization; ♦, presence of 0.135 M CaCl2 in reaction solution; ◂, immersion in 0.225 M CaCl2 aqueous solution after each cycle.
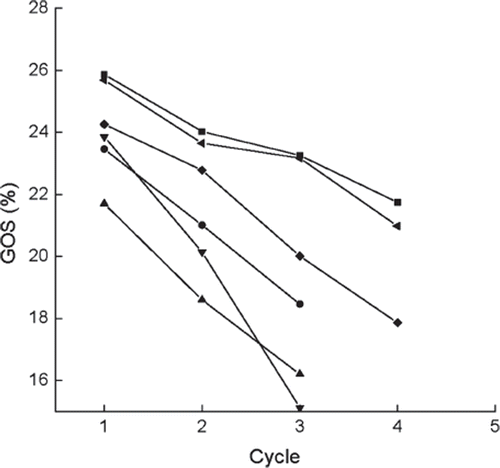
Table 1. Average yields of GOS over 10 cycles at different temperatures and pH values