Figures & data
Figure 1. Column bioreactor packed with yeast APA microcapsules (60% yeast APA microcapsules loading). The column bioreactor design criterion had the average surface area of a conventional dialyzer membrane, which is estimated to be 1.8 m2. The column bioreactor's height and internal diameter are 12 cm and 22 mm, respectively. The column bioreactor is made of Poly (methyl methacrylate) (plexiglass). Two identical meshes equipped with rubber O-ring were utilized to prevent the microcapsules from being drifted away by the flow.
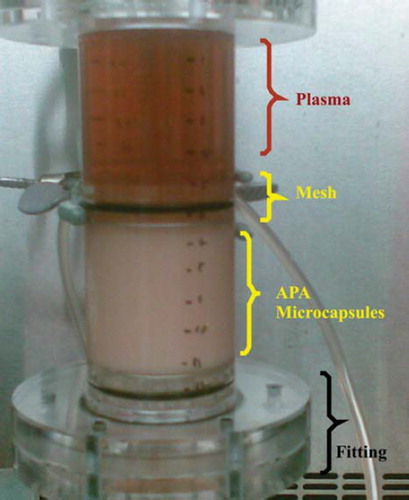
Figure 2. The efficacy of various yeast APA microcapsules loadings in plasma urea removal at a high flow rate 3.33 mL/min. The figure shows that higher the loading is, the greater the urea breakdown will be.
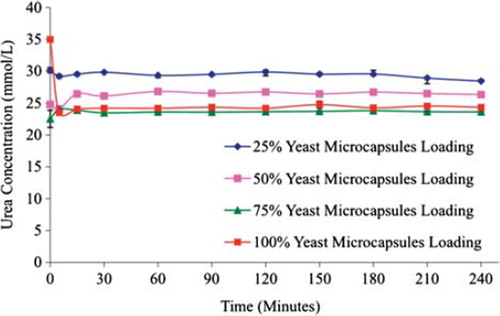
Figure 3. The effect of plasma flow rate through the column bioreactor on the urea removal by yeast APA microcapsules. The temperature of the re-circulating plasma was maintained at 37°C. 1 mL sample volume was retrieved at 0 min, 5 min, 15 min, 30 min, 1 hr, and after every 30 min interval thereafter until the 4th hour. (A) – Low flow rate (0.96 and 0.75 mL/min.) in 75% and 100% yeast APA microcapsules loading. (B) – Medium flow rate (2.19 and 1.55 mL/min.) in 75% and 100% yeast APA microcapsules loading. (C) – High flow rate (4.83 and 3.33 mL/min.) in 75% and 100% yeast APA microcapsules loading.
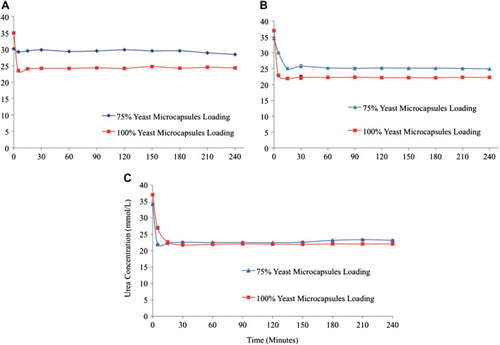
Figure 4. Comparative creatinine removal by 100 % yeast APA microcapsules loading at low, medium, and high flow rates (0.75, 1.55 and 3.33 mL/min, respectively). The temperature of the re-circulating plasma was maintained at 37°C. 1 mL sample volume was retrieved at 0 min, 5 min, 15 min, 30 min, 1 hr, and after every 30 min interval thereafter until the 4th hr.
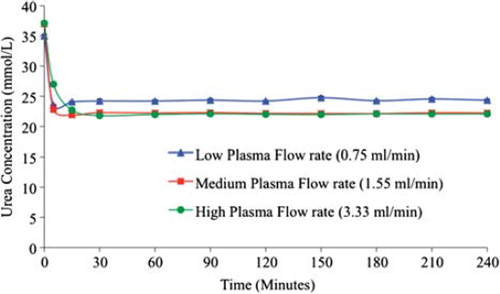
Figure 5. Percent change in the plasma urea (A), creatinine (B), phosphate (C), and ammonia (D) concentrations with 100 % yeast APA microcapsules loading at low, medium, and high flow rates (0.75, 1.55, 3.33 mL/min, respectively). The temperature of the recirculating plasma was maintained at 37°C. 1 mL sample volume was retrieved at 0 min, 5 min, 15 min, 30 min, 1 hr, and after every 30 min interval thereafter until the 4th hr.
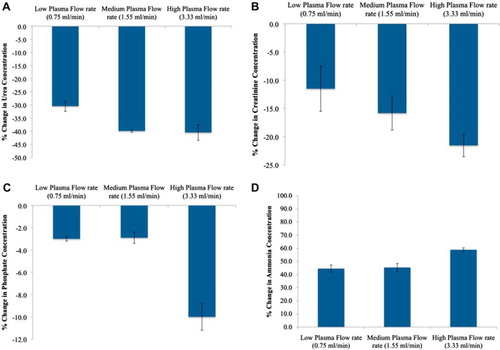
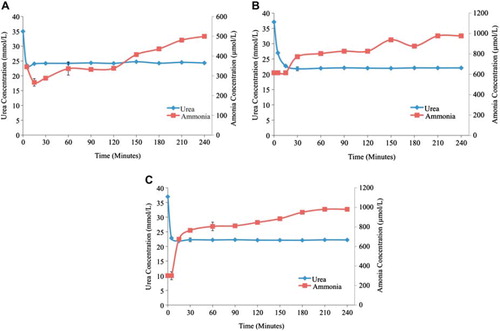
Figure 6. Plasma urea and ammonia concentrations as a function of time. (A) - Low flow rate (0.75 ml/min.) in 100% yeast APA microcapsules loading. (B) - Medium flow rate (1.55 ml/min.) in 100% yeast APA microcapsules loading. (C) - High flow rate (3.33 ml/min.) in 100% yeast APA microcapsules loading. The temperature of re-circulating plasma was maintained at 37°C using hot plates. The experiment lasted for 4 hrs. 1 ml sample volume was retrieved at 0min, 5min, 15min, 30min, 1hr, and after every 30 min interval until the 4th hour.