Figures & data
Table 1. Purification of pectinesterase enzyme isolated from Malatya apricot.
Table 2. Immobilization of PE from Malatya apricot on porous glass beads.
Table 3. Inactivation rate constants and of free and immobilized apricot PEs.
Figure 2. The effect of pH on the activity of free and immobilized apricot PE. Reactions were made with 0.5 mL 0.2 M phosphate buffer (pH 7.5) into 4 mL 1% pectin in 0.2 N NaCl.
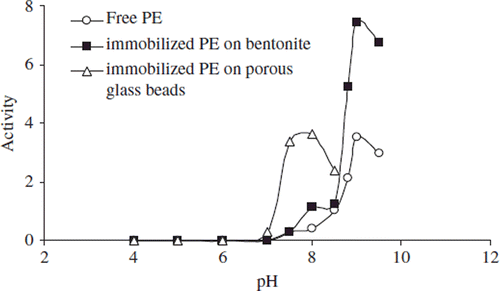
Figure 3. The effect of temperature on the activity of free and immobilized apricot PE. Reactions were made with 1% pectin concentration at pH 9.0.
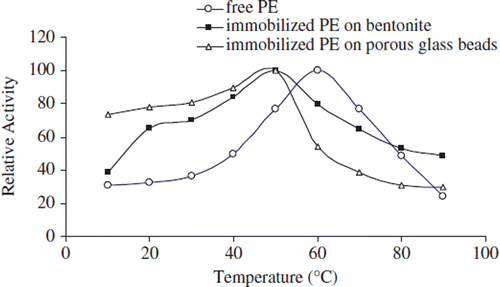
Figure 4. The thermal stability profıle for free apricot PE. Reactions were made with 0.5 mL enzyme in 0.2 M phosphate buffer (pH 7.5) into 4 mL 1% pectin in 0.2 N NaCl at pH 9.0.
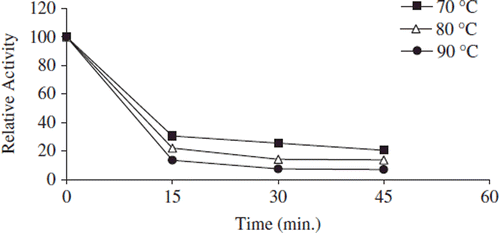
Figure 5. The thermal stability profıle for covalently immobilized apricot PE. Reactions were made with 1% pectin concentration and 0.05 g immobilized enzyme dissolved with 0.5 mL 0.2 M sodium phosphate buffer (pH 7.5) at pH 9.0.
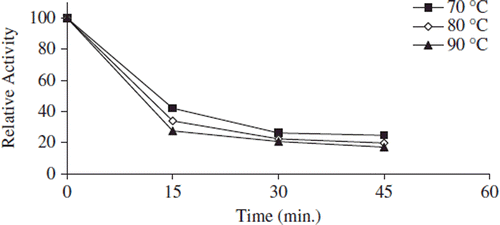
Figure 6. The thermal stability profıle for noncovalently immobilized apricot PE. Reactions were made with 1% pectin concentration and 0.05 g immobilized enzyme dissolved with 0.5 mL 0.2 M sodium phosphate buffer (pH 7.5) at pH 9.0.
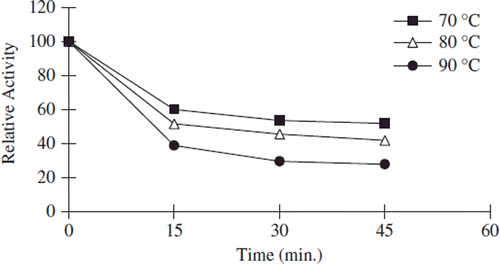
Table 4. Kinetic properties of free and covalently and noncovalently-immobilized apricot PEs.