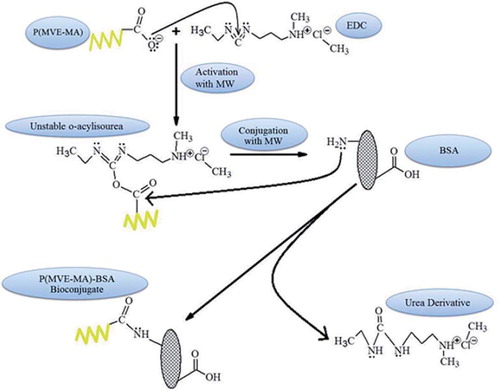
Figures & data
Table I. The experimental ratios and concentrations of nBSA/nP(MVE-MA) [BSA Mw:66 kDa and P(MVE-MA) Mw:70 kDa].
Figure 1. Conventional Method Conjugates (CMC): HPLC chromatograms of BSA, P(MVE-MA), and BSA-P(MVE-MA) conjugates prepared at ratio on nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
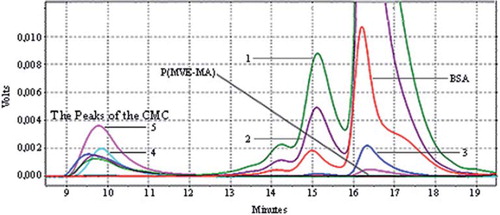
Figure 2. Microwave Assisted Conjugates (MWC): HPLC chromatograms of BSA, P(MVE-MA), and BSA-P(MVE-MA) conjugates prepared at ratio on nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
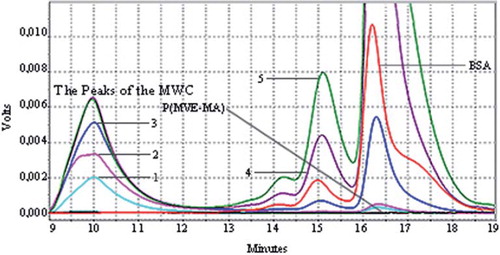
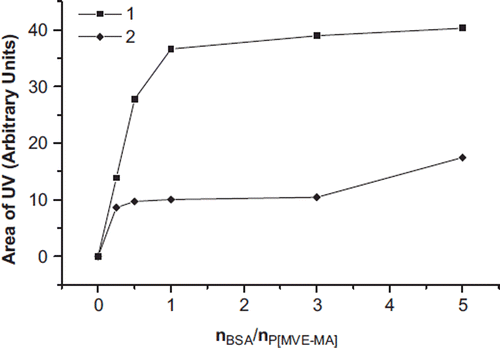
Figure 3. SEC [UV (a), LS (b)] chromatograms of the conjugates by conventional method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
![Figure 3. SEC [UV (a), LS (b)] chromatograms of the conjugates by conventional method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).](/cms/asset/1a9cf134-a7e7-47dc-a951-534ff707990e/ianb19_a_678942_f0003_b.jpg)
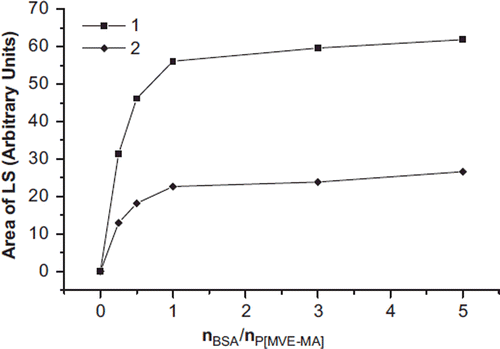
Figure 4. SEC [UV (a), LS (b)] chromatograms of the conjugates by means of the microwave-assisted method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
![Figure 4. SEC [UV (a), LS (b)] chromatograms of the conjugates by means of the microwave-assisted method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).](/cms/asset/ba4587f6-9f98-4320-9cef-4d6abe4eac7e/ianb19_a_678942_f0004_b.jpg)