Figures & data
Figure 1. Intracellular and extracellular proteolytic targeting of articular cartilage. (A) Illustration of an articular chondrocyte embedded in extracellular matrix composed of HA, aggrecan and collagen type II fibrils. Upon pro-catabolic stress, proteolytic enzymes as MMPs and ADAMTS are released to the extracellular space via exocytotic vesicles, while lysosomal cathepsins are also found throughout the cellular compartments, due to leaky lysosomes. One cellular protein target of cathepsins is SIRT1 which is cleaved (i.e. 75SIRT1) and exported from the nucleus under proinflamtory conditions, as also reported for Bid (Cirman et al., Citation2004; Dvir-Ginzberg et al., Citation2011). (B) Collagen type II cleavage by cathepsins (white scissors), MMP (grey scissors). MMPs target the collagen type II triple helix, generating Helix-II, C2C, Coll2-1NO2/Coll2-1 and C1,C2 neo-peptides (Dejica et al., Citation2012; Rousseau & Delmas, Citation2007). Cathepsins are capable of cleaving the collagen type II N-telopeptide (i.e. generating C2K, PIINP and PIIANP) and C-telopeptide (i.e. generating CTX-II neopeptide fragments) (Dejica et al., Citation2012; Rousseau & Delmas, Citation2007). (C) ADAMTS (striped scissors) mediated cleavage of aggrecan between the G1/G2 domains and several regions between the G2 and G3 which are glycosylated with keratin sulfate (KS) and chondroitin sulfate (CS) (Fosang et al., Citation2008). Cathepsin (B and D; blue scissors) additionally cleave G1/G2 domains and targets chondroitin sulfate attachment regions (Handley et al., Citation2001; Mort et al., Citation1998). (D) Linear hyaluronic acid is cleaved by hyaluronidase (HAse; black scissors) at the β(1,4) site between N-acetyl-d-glucosamine and d-gluroniuc acid leading to smaller and random fragments which reduce the overall molecular weight of the HA mesh within articular cartilage.
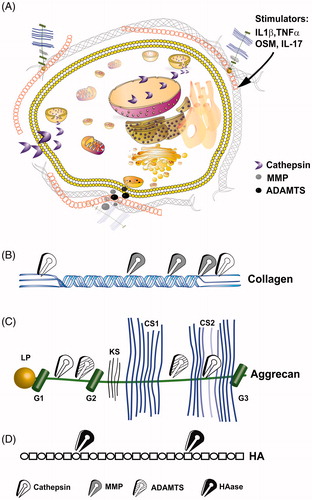
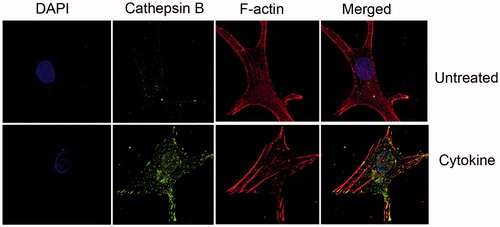
Figure 2. Increased cellular cathepsin during cytokine treatment of human chondrocytes. OA human-derived chondrocytes were obtained following an informed consent from individuals undergoing total knee replacement (Hadassah Institutional approval obtained). Chondrocytes were isolated and cultured, treated (Cytokine: 50 ng/ml TNFα+2 ng/ml IL1β, 24 h) and stained as previously described (Dvir-Ginzberg et al., Citation2011; Oppenheimer et al., Citation2012). Cells stained with DAPI (i.e. nuclei in blue fluorescence), cathepsin B (i.e. active cathepsin B in green florescence), Phalloidine (i.e. F-actin filaments in red florescence), × 600 magnification. The images clearly show that cytokine treatment renders increased cathepsin B levels throughout the cell compartment, as well as the nucleus. The micrographs are representative images of data previously described by Oppenheimer et al., Arthritis and Rheumatology (Oppenheimer et al., Citation2012).