Figures & data
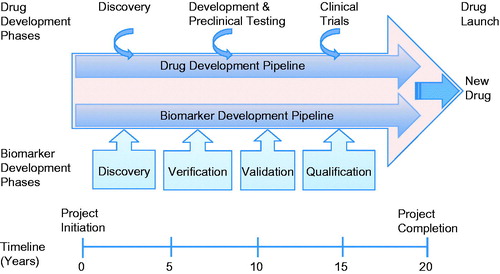
Figure 1. Applying the biomarker toolbox in the drug discovery and development pathway. This schematic highlights the mutual interdependency of the drug and biomarker development pipelines. Linking a biomarker to a complementary endpoint facilitates the drug discovery process and allows pharmaceutical companies to make rational decisions about the continuity of preclinical studies and clinical trials. Biomarkers can be used at critical decision points to make go/no-go decisions. They can also be used in translational research, bridging the gap between the bench and the bedside. Biomarkers can also be used to identify responders and non-responders and quantify clinical efficacy and patient stratification (i.e. identification of those in need of treatment and selection of patients most likely to respond to treatment). In phase II clinical trials biomarkers can be used for dose determination and safety/efficacy studies. They can also help pharmaceutical companies save costs by enabling drug repositioning and determining the cost/benefit ratio for treatment. In routine clinical practice biomarkers are important diagnostic and prognostic tools for monitoring disease development and monitoring patients compliance. They are also indispensable tools for pharmacovigilance, personalized and precision health care and differentiating compounds from competitors.