Figures & data
Figure 1. Patient flow through the trial. NOMAC, nomegestrol acetate; E2, oestradiol; EE, ethinylestradiol; DRSP, drospirenone; AE, adverse event.
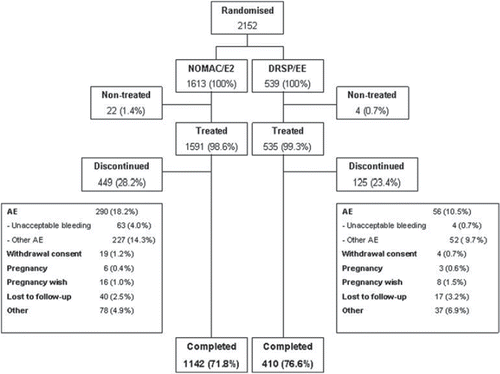
Table 1 Demographic characteristics of subjects at screening
Table 2 Summary of contraceptive efficacy (Pearl Index and life-table analysis) for nomegestrol acetate/17β-oestradiol (NOMAC/E2) and drospirenone/ethinylestradiol (DRSP/EE) in the overall population and age subgroups
Figure 2. Mean number of bleeding-spotting days per 91-day reference periods for (A) NOMAC/E2 and (B) DRSP/EE. NOMAC, nomegestrol acetate; E2, oestradiol; EE, ethinylestradiol; DRSP, drospirenone.
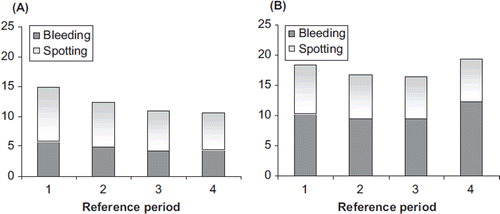
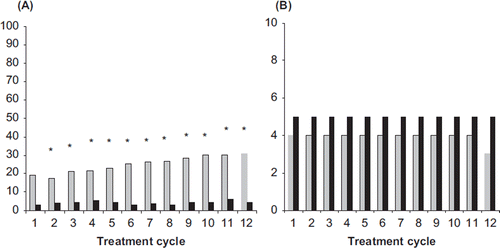
Figure 3. (A) Incidences (%) and (B) duration (median number of days) of breakthrough bleeding-spotting for NOMAC/E2 (grey bars) and DRSP/EE (black bars). The incidences were compared statistically between the treatment groups. *p < 0.05 vs. DRSP/EE. NOMAC, nomegestrol acetate; E2, oestradiol; EE, ethinylestradiol; DRSP, drospirenone.
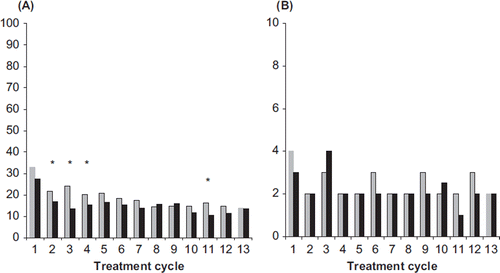
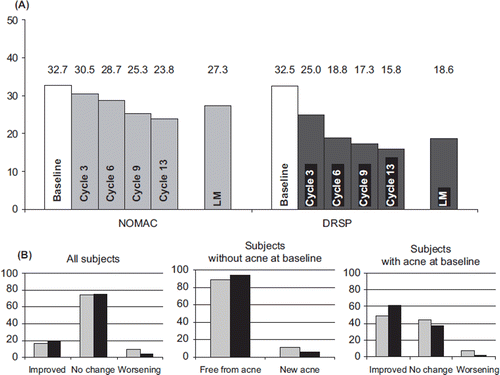
Figure 4. (A) Incidences of absence of withdrawal bleeding (%) and (B) duration of withdrawal bleeding (median number of days) for NOMAC/E2 (grey bars) and DRSP/EE (black bars). The incidences were compared statistically between the treatment groups. *p < 0.05 vs. DRSP/EE. NOMAC, nomegestrol acetate; E2, oestradiol; EE, ethinylestradiol; DRSP, drospirenone.