Figures & data
Figure 1. Summarized timelines comparing VOLT and VERSUS. VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US; PPBC, Patient Perception of Bladder Condition; OAB-q, Overactive Bladder Questionnaire; OAB, overactive bladder.
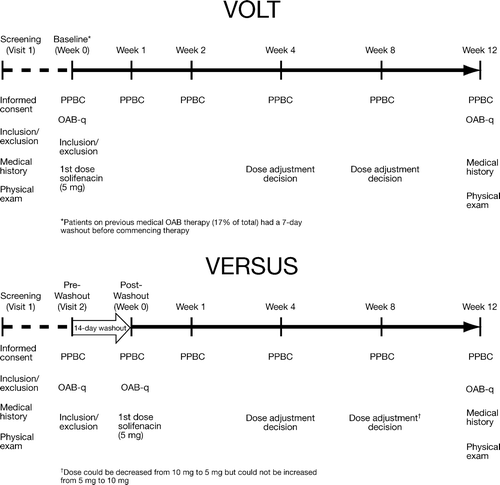
Table I. Baseline demographics.
Figure 2. Distribution of male patients by PPBC score at baseline (VOLT, top) or Post-Washout (VERSUS, bottom) and end of treatment for both studies. PPBC, Patient Perception of Bladder Condition; VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US.
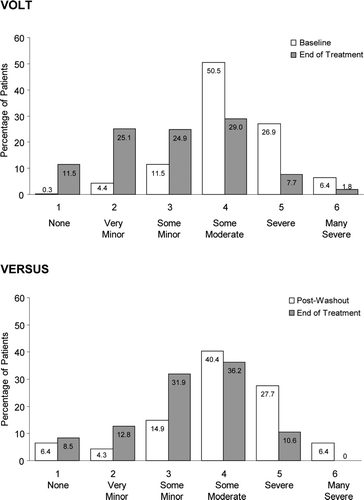
Figure 3. Change from baseline for OAB-q scores in men from VOLT and VERSUS. For the Symptom Bother scale, a negative value indicates improvement; for the HRQL scale and domains, a positive value indicates improvement. All changes from baseline were statistically significant (p < 0.001) as demonstrated using the two-sided, 1-sample t-test (α = 0.05). Scores shown from VERSUS are Post-Washout, after tolterodine cessation. HRQL, health-related quality of life; OAB-q, Overactive Bladder Questionnaire; VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US.
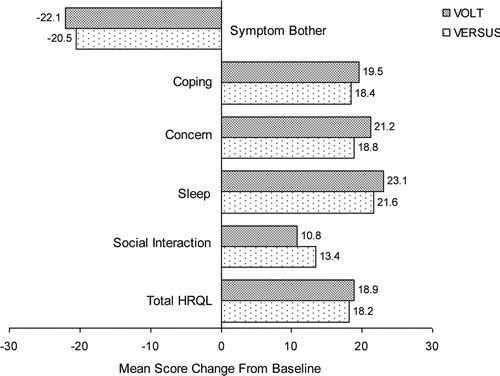
Figure 4. Results for diary-based endpoints in men from VERSUS: median percentage changes from baseline to study end (bar graph) and summary of mean values (table). BL, baseline; EOT, end of treatment; VERSUS, VESIcare Efficacy and Research Study US.
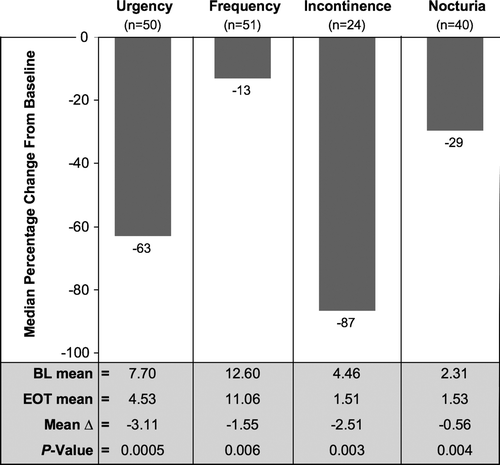
Table II. Treatment-emergent adverse events occurring in >1% of subjects in VOLT and VERSUS.