Figures & data
Table I. Values for TT, FT, BT, SHBG, and total PHQ-9 scores at baseline and 3, 6, and 12 months of TRT.
Figure 1. Distribution of patients at baseline with depression symptoms ranging from none to severe. Symptom severity was determined using the total PHQ-9 score for each patient. The mean total testosterone level for each degree of symptom severity is listed below the graph. PHQ-9 = Patient Health Questionnaire-9.
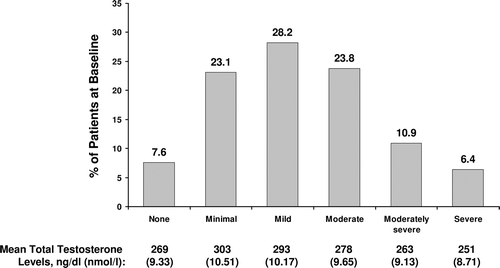
Figure 2. Percentage of patients with moderately severe to severe depression at baseline in the overall population and by subgroup. Subgroups with significantly more patients with moderately severe to severe depression were characterized by antidepressant use, self-reported depression history, age, baseline total testosterone level, opioid use, and HIV/AIDS. HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; PDE5I = phosphodiesterase type 5 inhibitors; TT = total testosterone.
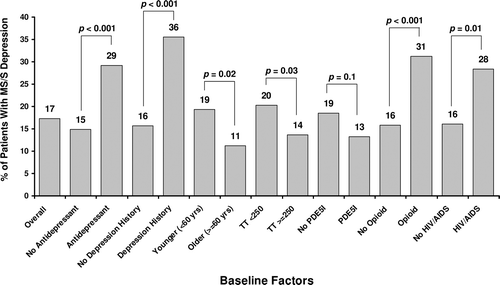
Figure 3. Change in total testosterone levels (A) and total PHQ-9 scores (B) after initiation of TRT. *p < 0.01 versus baseline. PHQ-9 = Patient Health Questionnaire-9; TRT = testosterone replacement therapy; TT = total testosterone.
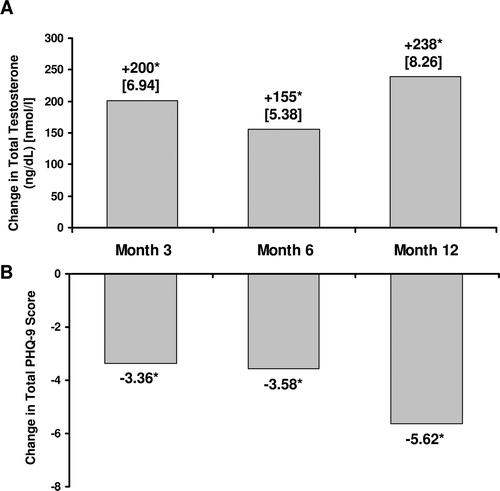
Figure 4. Reduction in the percentages of patients with PHQ-9 scores denoting mild/moderate and moderately severe/severe depression symptoms, and increase in the percentage of patients with no/minimal depression symptoms, after initiation of TRT. The denominator within each bar is the number of patients available for PHQ-9 testing at that time point. Significant improvements were seen for all patient groups at all time points (p < 0.0001), though maximal improvement was not seen until 12 months of TRT. PHQ-9 scores: No or minimal depression symptoms, <5; mild to moderate depression symptoms, 5–14; moderately severe to severe depression symptoms, 15–27. PHQ-9 = Patient Health Questionnaire-9; TRT = testosterone replacement therapy; MS/S = moderately severe to severe depression.
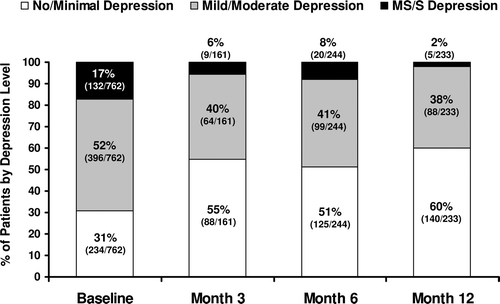
Table II. Baseline values and change in PHQ-9 scores from baseline, total and by question.
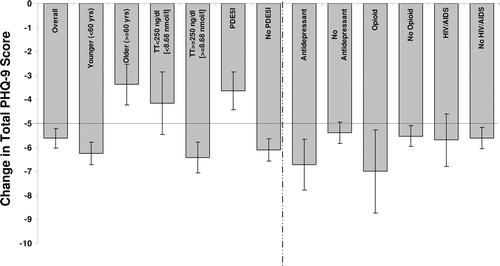
Figure 5. Change in total PHQ-9 scores (mean ± SE) after 12 months TRT in the overall population and in subgroups. A decrease of ≥5 points (dotted line) represents a meaningful clinical response to treatment. Subgroups with significantly different responses were characterized by age, baseline total testosterone level, and PDE5 inhibitor use (bars to the left of the dashed line). PHQ-9 = Patient Health Questionnaire-9; SE = standard error; TRT = testosterone replacement therapy; TT = total testosterone.