Figures & data
Table 1. Baseline characteristics.
Figure 1. Mean ± standard error (SE) total International Prostate Symptom Score (IPSS) over time for testosterone undecanoate 80–240 mg/day versus placebo.
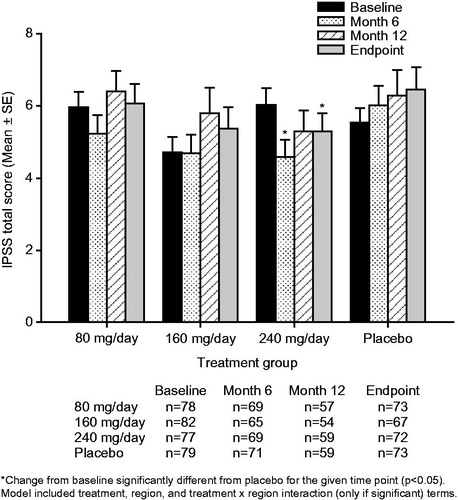
Figure 2. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) quality of life subscore over time for testosterone undecanoate 80–240 mg/day versus placebo. Decrease in score = improvement.
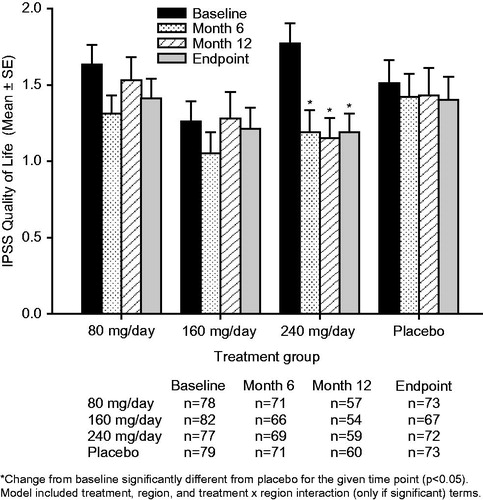
Figure 3. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) storage subscore over time for testosterone undecanoate 80–240 mg/day versus placebo.
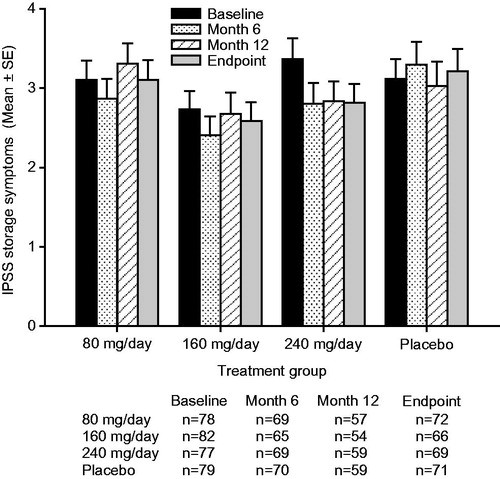
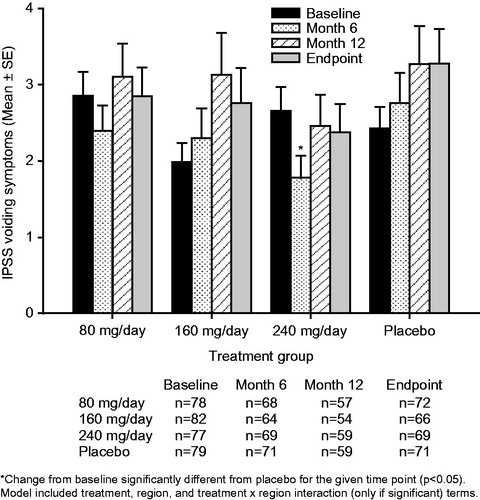
Figure 4. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) voiding subscore over time for testosterone undecanoate 80–240 mg/day versus placebo.