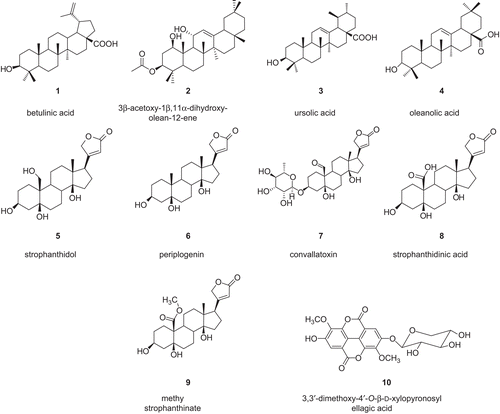
Figures & data
Figure 2. Free radical scavenging activity of the methanol extract, compounds from Antiaris africana, and vitamin C: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 3, ursolic acid; 4, oleanolic acid; 5, strophanthidol; 6, periplogenin; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
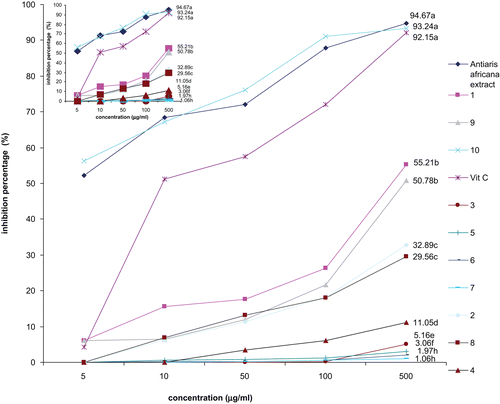
Figure 3. Antitumor activity of the methanol extract and compounds from Antiaris africana as obtained in Crown gall assay: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 3, ursolic acid; 4, oleanolic acid; 5, strophanthidol; 6, periplogenin; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
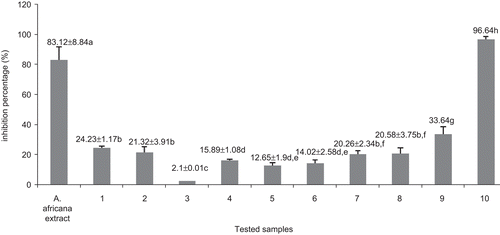
Figure 4. Effects of the methanol extract and compounds from Antiaris africana on (A) DU-145 and (B) Hep G2 cell survivals: values with the same letter are not significantly different (ANOVA, p < 0.05). 1, betulinic acid; 2, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene; 7, convallatoxin; 8, strophanthidinic acid; 9, methylstrophanthinate; 10, 3,3′-dimethoxy-4′-O-β-d-xylopyronosylellagic acid.
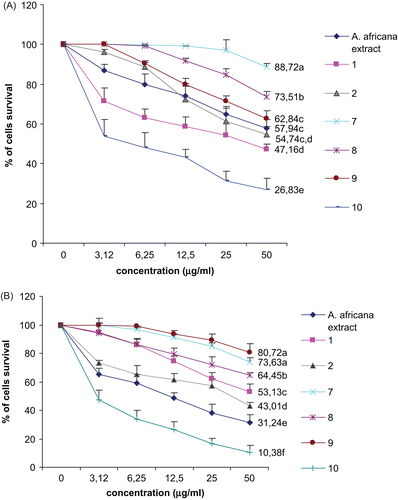
Table 1. Activity of the crude extract from A. africana, selected compounds, and doxorubicin on human cancer cell lines DU-145 and Hep G2.