Figures & data
Figure 1. (A) Saturation curve for the 5-hydroxytryptamine1A receptor with [3H]8-OH-DPAT as the radioligand. Receptor membrane (35 μg protein/well) was incubated with increasing concentrations of [3H]8-OH-DPAT (0–10 nM) at 25°C for 90 min. The binding was fitted to the two-site binding model. The Rosenthal plot is shown as the inset of the saturation binding curve. (B) Time course of association of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.2 nM) at 25°C for different time periods (0–180 min). Each data point is expressed as mean ± SD (n = 3).
![Figure 1. (A) Saturation curve for the 5-hydroxytryptamine1A receptor with [3H]8-OH-DPAT as the radioligand. Receptor membrane (35 μg protein/well) was incubated with increasing concentrations of [3H]8-OH-DPAT (0–10 nM) at 25°C for 90 min. The binding was fitted to the two-site binding model. The Rosenthal plot is shown as the inset of the saturation binding curve. (B) Time course of association of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.2 nM) at 25°C for different time periods (0–180 min). Each data point is expressed as mean ± SD (n = 3).](/cms/asset/872603c4-7f92-4aa6-91e5-f759035eb12c/iphb_a_401040_f0001_b.gif)
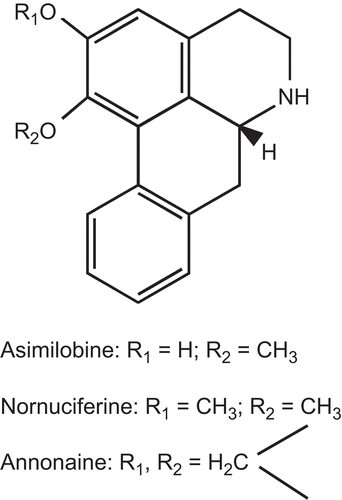
Figure 2. Chemical structures of compounds isolated from P. odoardi as competitive inhibitors on [3H]8-OH-DPAT binding to 5-hydroxytryptamine1A receptor.
![Figure 2. Chemical structures of compounds isolated from P. odoardi as competitive inhibitors on [3H]8-OH-DPAT binding to 5-hydroxytryptamine1A receptor.](/cms/asset/8541aaff-faf8-459a-a261-193b3c3aa59b/iphb_a_401040_f0002_b.gif)
Figure 3. Competitive inhibition on the binding of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor by O-methyldauricine and popisidine. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.4 nM) in the presence of O-methyldauricine and popisidine at 25°C for 90 min. Each data point is expressed as mean ± SD (n = 3).
![Figure 3. Competitive inhibition on the binding of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor by O-methyldauricine and popisidine. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.4 nM) in the presence of O-methyldauricine and popisidine at 25°C for 90 min. Each data point is expressed as mean ± SD (n = 3).](/cms/asset/5d45ebfc-1c64-43f4-a6d2-b528a34749aa/iphb_a_401040_f0003_b.gif)
Table 1. Ki values obtained from the competition experiments with standard unlabelled ligands on 5-HT1A receptor binding activity with [3H]8-OH-DPAT as the radioligand.