Figures & data
Table 1. Hemagglutinating activities and yields at various stages of purification of lectin from Parkia speciosa.
Figure 1. Affinity chromatogram of crude protein of Parkia speciosa on Affi-Gel Blue gel column (1.6 × 10 cm) in binding buffer 1 mM Tris-HCl, pH 7.2. Dashed lines indicate use of 0–100% linear gradient of 0.5 mM NaCl in binding buffer. Flow rate 1.5 mL/min.
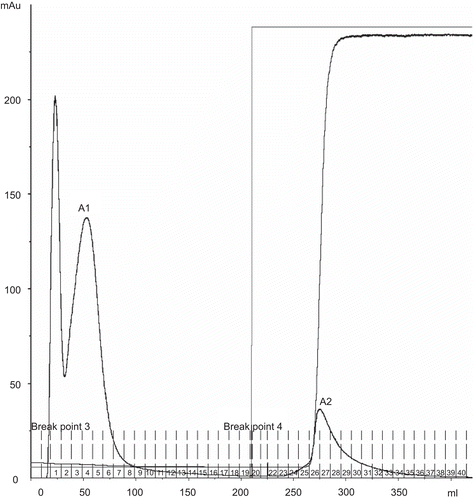
Figure 2. Gel filtration chromatogram of A1, A2 fractions from Affi-Gel Blue gel chromatography on Superdex 200 column (1.6 × 60 cm) in double distilled water. Flow rate 0.5 mL/min.
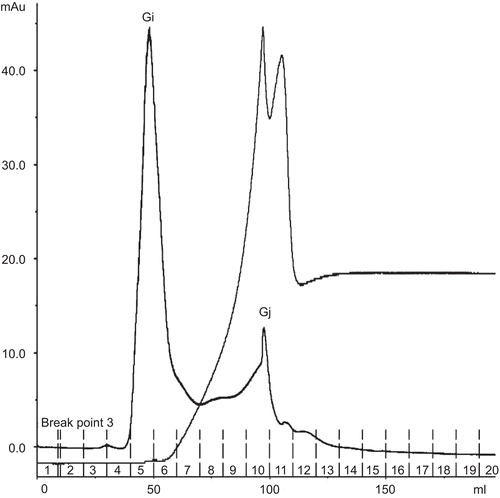
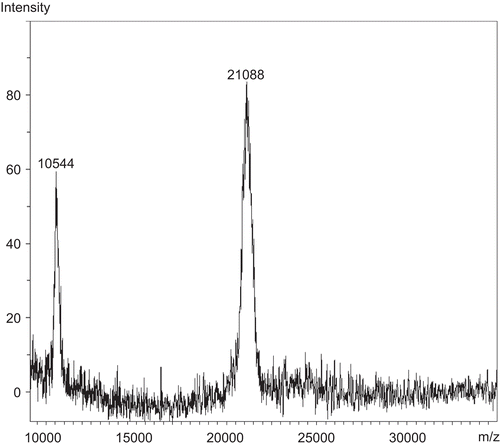
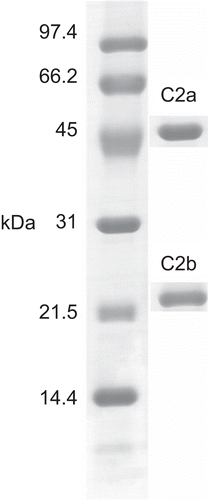
Table 2. Proteins identified by MALDI-ToF mass spectrometry followed by peptide mass fingerprinting via the Mascot search engine.
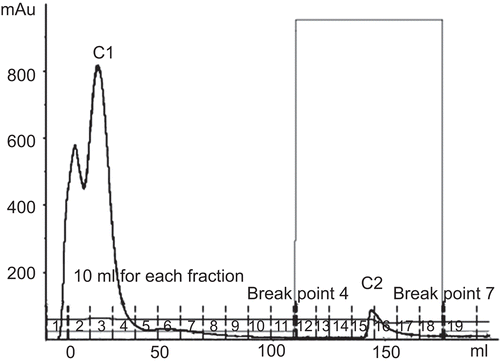
Figure 4. Affinity chromatogram of crude protein from Parkia speciosa on Con A Sepharose column (1.6 × 5 cm). Flow rate 1.5 mL/min. The equilibration buffer was 20 mM Tris-HCl, pH 7.4, containing 0.5 M NaCl, 1 mM CaCl2·2H2O, 1 mM MnCl2. The eluted lectin buffer was 20 mM Tris-HCl, pH 7.4, containing 0.1–0.5 M methyl-α-d-mannopyranoside.