Figures & data
Table 1. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced hepatotoxicity in albino rats.
Table 2. Antioxidant activity of Stereospermum suaveolens against CCl4-induced hepatotoxicity.
Figure 1. Histopathological observations of normal group rat liver (A), showing normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes, and no dilation of portal vein. The control (CCl4-treated) group rat liver (B), exhibited intense centrilobular necrosis, vacuolization, macrovesicular fatty changes and distorted central vein architecture. The positive control group rat liver (C) also restores the normal histopathological observations.
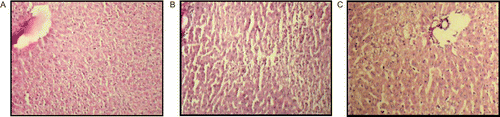
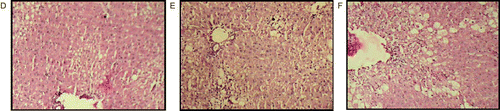
Figure 2. Histopathological observations of dose125 mg/kg treated group (D), 250 mg/kg treated group (E) and 500 mg/kg of extract-treated groups showed almost normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes and no dilation of portal vein, but high dose (500 mg/kg) treated group showed some vacuolization and dilation of hepatic vein.