Figures & data
Figure 1. The gradient high-performance liquid chromatography (HPLC) chromatogram of (A) cold water crude extract of Terminalia chebula galls containing phenolic compounds including gallic acid (1), 1,3,6-tri-O-galloyl-ß-d-glucopyranose (2), punicalagin (3), isoterchebulin (4), chebulagic acid (5), and chebulinic acid (6) and (B) the semipurified fraction.
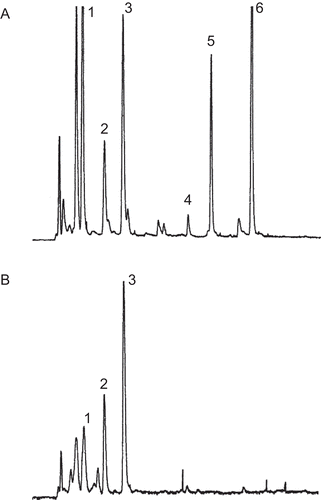
Table 1. Percentage yields and total phenolic contents of fractions by column chromatography from the cold aqueous Terminalia chebula gall crude extract.
Table 2. Size, entrapment efficiency, zeta potential, and deformability index (DI) of nonelastic and elastic niosomes loaded with gallic acid or the semipurified fraction containing gallic acid.
Figure 2. Negative-staining TEM images of elastic and nonelastic niosomes (Tween 61 mixed with cholesterol at 1:1 molar ratio, 20 mM) loaded with gallic acid and the semipurified fraction containing gallic acid: (A) elastic niosomes loaded with gallic acid (GE) (×15,000); (B) nonelastic niosomes loaded with gallic acid (GN) (12,000×); (C) elastic niosomes loaded with the semipurified fraction (SE) (15,000×); and (D) nonelastic niosomes loaded with the semipurified fraction (SN) (15,000×).
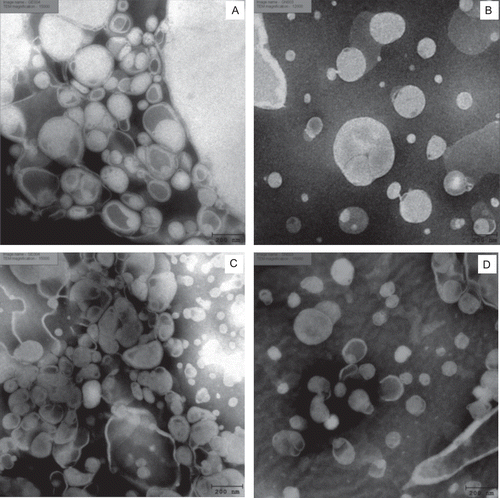
Figure 3. The percentages of gallic acid remaining in various formulations at different storage temperatures (27 ± 2, 4 ± 2, and 45 ± 2°C) for 3 months. GS = gallic acid in phosphate buffer solution; GE = elastic niosomes loaded with gallic acid; GN = nonelastic niosomes loaded with gallic acid; SS = the semipurified fraction in phosphate buffer solution; SE = elastic niosomes loaded with the semipurified fraction; SN = nonelastic niosomes loaded with the semipurified fraction.
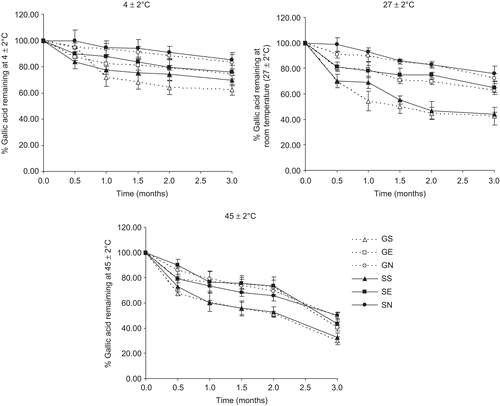
Figure 4. Cumulative amounts (µg/cm2) of gallic acid from various gel formulations versus time (hours) in stratum corneum (SC) (A), viable epidermis and dermis (VED) (B) and receiving solution (C) following transdermal absorption across excised rat skin by vertical Franz diffusion cells. ![]()
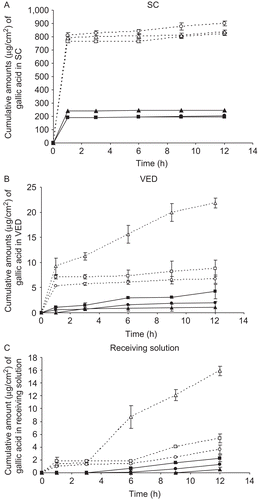
Table 3. The percentages of gallic acid amounts (%) in stratum corneum (SC), viable epidermis and dermis (VED), and receiving solution following transdermal absorption across excised rat skin from various gel formulations by Franz diffusion cells after 12 h.