Figures & data
Table 1. Composition of GGEx18.
Table 2. Sequences of primers used for the RT-PCR assays.
Figure 1. GGEx18 inhibits high fat diet–induced body weight gain and total adipose tissue mass in C57BL/6J mice. Adult male C57BL/6 mice were fed a low-fat diet (normal), a high-fat diet (control), or the fat diet supplemented with 125, 250, and 500 mg/kg/day GGEx18 for 9 weeks. (A) Body weights at the end of the treatment period were statistically significant between normal and control groups (p < 0.05), and between the control group and the 250 mg/kg/day and 500 mg/kg/day GGEx18 groups (p < 0.05). (B) At the end of study, adipose tissue weights were measured. All values are expressed as the mean ± SD. #p < 0.05 compared with normal group, *p < 0.05 compared with control group. Normal, low fat diet-fed mice; control, high fat diet-fed mice; GGEx18, GGEx18-treated high fat diet-fed mice.
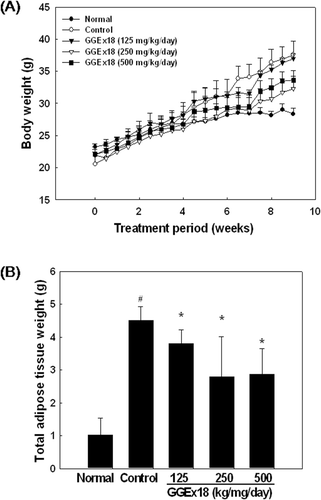
Figure 2. GGEx18 inhibits high fat diet–induced increases in lipid accumulation in skeletal muscle of C57BL/6J mice. Adult male C57BL/6J mice were fed a low-fat diet (normal), a high-fat diet (control), or the high-fat diet supplemented with 125, 250, and 500 mg/kg/day for 9 weeks. (A) Histology showing transverse sections of skeletal muscle. (B) Higher magnitude of bracket area from (A). Shown are representative oil red O-stained sections (7-μm thick) of skeletal muscle. The number and intensity of stained fibers in the GGEx18 group were much less than that in the control group. Normal, low fat diet-fed mice; control, high fat diet-fed mice; GGEx18, GGEx18-treated high fat diet-fed mice.
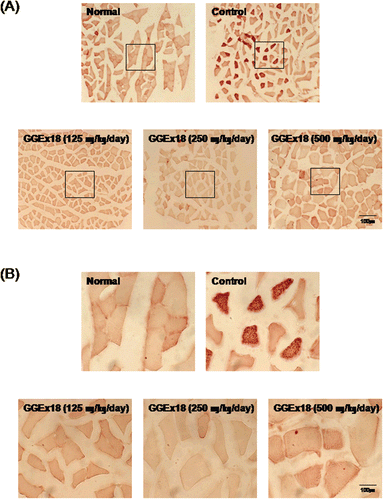
Figure 3. GGEx18 increases the mRNA expression levels of AMPK, PPARα, and PPARα target genes in skeletal muscle of C57BL/6J mice. (A) Adult male C57BL/6J mice were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 250 mg/kg/day GGEx18 for 9 weeks. Total cellular RNA was extracted from skeletal muscle tissue, and mRNA levels were measured using RT-PCR. All values are expressed as the mean ± SD of relative density units using β-actin as a reference. *p < 0.05 compared with control group. (B) Representative RT-PCR bands from one of the three independent experiments are shown. Control, high fat diet–fed mice; GGEx18, GGEx18 (50 mg/kg/day)-treated high fat diet–fed mice.
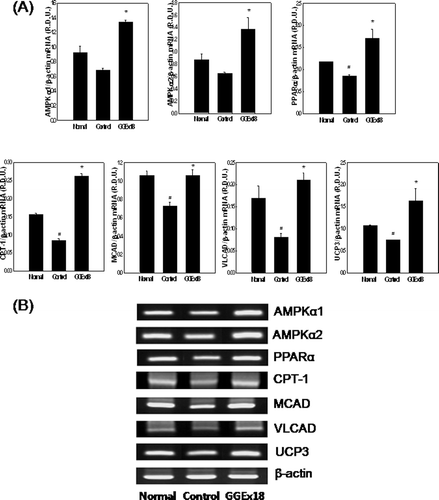
Figure 4. GGEx18 decreases triglyceride droplets in C2C12 cells. C2C12 cells were differentiated as described in the Materials and Methods section and triglycerides were stained with oil red O. (A) Non-differentiated cells (ND). Differentiated cells treated with (B) DMSO, (C) 10 μg/mL GGEx18, (D) 10 µM fenofibrate, and (E) 10 µM Wy14,643.
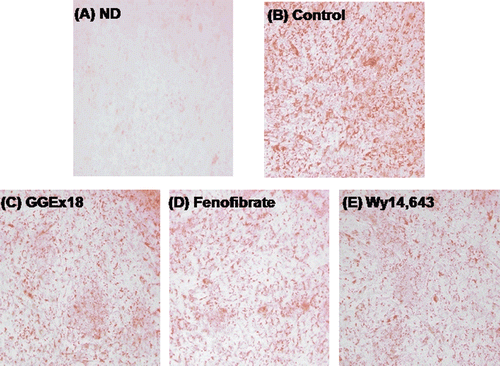
Figure 5. Compound C increases GGEx18-induced decreases in triglyceride droplets in C2C12 cells. C2C12 cells were differentiated as described in the Materials and Methods section and triglycerides were stained with oil red O. Differentiated cells treated with (A) DMSO, (B) 10 μg/mL GGEx18, (C) 10 μg/mL GGEx18 plus 0.1 μM compound C, (D) GGEx18 plus 1 μM compound C, and (E) GGEx18 plus 10 μM compound C. Comp C, compound C.
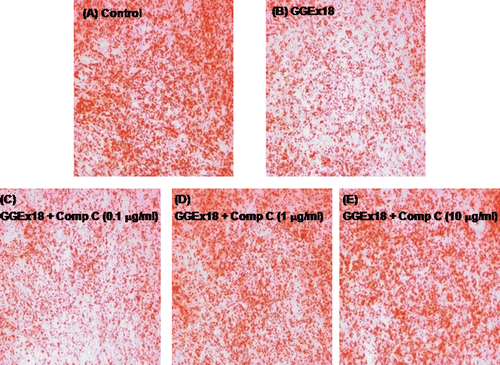
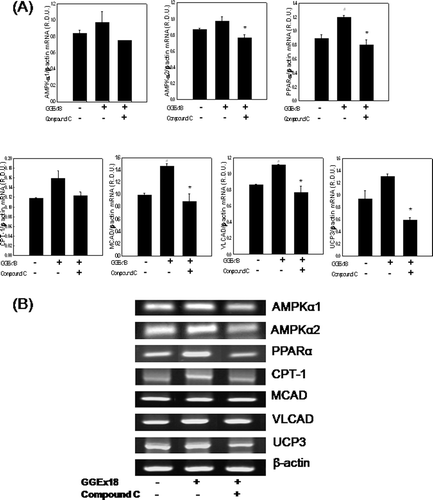
Figure 6. Compound C decreases GGEx18-induced increases in mRNA expression levels of AMPK, PPARα, and PPARα target genes in C2C12 cells. C2C12 cells were differentiated as described in the Materials and Methods section. C2C12 cells were treated with DMSO, 10 μg/mL GGEx18, and 10 μg/mL GGEx18 plus 1 µM compound C. Total cellular RNA was extracted from differentiated cells and mRNA levels were measured using transcription-polymerase chain reaction (RT-PCR). All values are expressed as the mean ± SD of relative density units using β-actin as a reference. #p < 0.05 compared with DMSO group, **p < 0.05 compared with GGEx18 group. (B) Representative RT-PCR bands from one of the three independent experiments are shown.