Figures & data
Table 1. In vitro antileishmanial and antiplasmodial activities and cytotoxicity of crude extracts and isolated compounds (1–7) from P. excelsa.
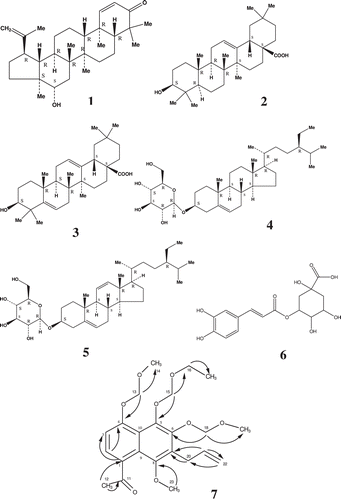
Table 2. 1H, 13C, COSY and HMBC NMR spectral data of 7.
Figure 1. 16β-Hydroxylupane-1,20(29)-dien-3-one (1), (3-β)-3-hydroxyolean-12-en-28-oic acid (2), 3β-hydroxy-olean-5,12-dien-28-oic acid (3), Daucosterol (4), 3-O-β-d-glucopyranosyl-stigmasta-5,11(12)-diene (5), chlorogenic acid (6) and 1-(7-allyl-5-(ethoxymethoxy)-8-methoxy-4,6-bis(methoxymethoxy)naphthalen-1-yl)ethanone (7). Black arrow: HMB correlation (2JC-H and 3JC-H).