Figures & data
Table 1. PCR primers and conditions used for cDNA synthesis by RT-PCR.
Figure 1. Effects of Gyeongshingangjeehwan 18 (GGEx18) on hepatic lipid accumulation in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. (A) Representative hematoxylin and eosin-stained sections of livers are shown (original magnification, ×100). (B) Histological analyses of hepatic lipid accumulation. Pathological scores of hepatic lipid accumulation are as follows: 0, no lesion; 1, mild; 2, moderate; 3, severe; 4, very severe. All values are expressed as the mean ± standard deviation of four independent experiments. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group.
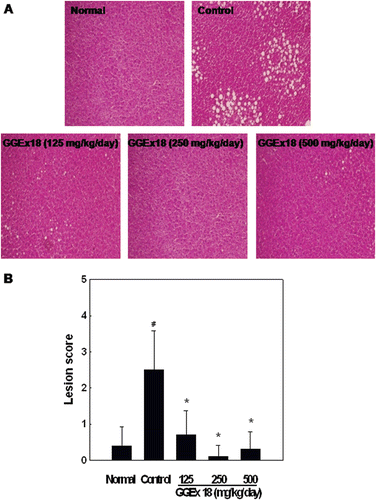
Figure 2. Effects of Gyeongshingangjeehwan 18 (GGEx18) on organ weights, serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. (A) Organ weights. (B) Serum ALT levels. (C) Serum AST levels. All values are expressed as the mean ± standard deviation. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group.
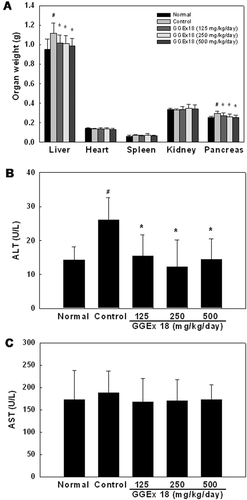
Figure 3. Effects of Gyeongshingangjeehwan 18 (GGEx18) on circulating (A) triglycerides and (B) total cholesterol in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. Serum concentrations of triglycerides and total cholesterol were measured, and all values are expressed as the mean ± standard deviation. #p < 0.05 compared with the normal group, *p <0.05 compared with the control group.
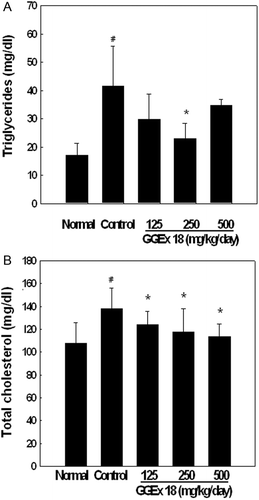
Figure 4. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα target gene mRNA levels in high-fat diet-induced obese mice. (A)Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 250 mg/kg/day GGEx18 for 9 weeks. Total cellular RNA was extracted from liver tissue, and mRNA levels were measured using RT-PCR. Data are reported as the mean ± standard deviation of relative density units (R.D.U.) using β-actin as a reference. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group. (B) Representative RT-PCR bands from one of four independent experiments are shown.
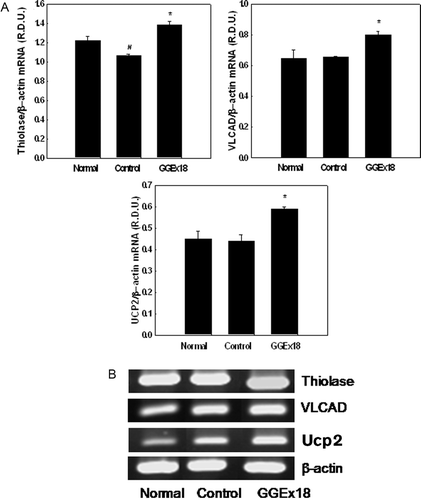
Figure 5. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα target gene mRNA levels in NMu2Li liver cells. (A) NMu2Li cells were transiently transfected with pSG5-mPPARα, the reporter plasmid PPRE-TK-Luc, the β-galactosidase gene, and pBSK. Cells were treated with GGEx18 at concentrations of 0.1, 1, and 10 μg/mL. After incubation for 24 h, cells were harvested. RNA was extracted from cells, and mRNA levels were measured using RT-PCR. Data are reported as the mean ± standard deviation of relative density units (R.D.U.) using β-actin as a reference. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group. (B) Representative RT-PCR bands from one of four independent experiments are shown.
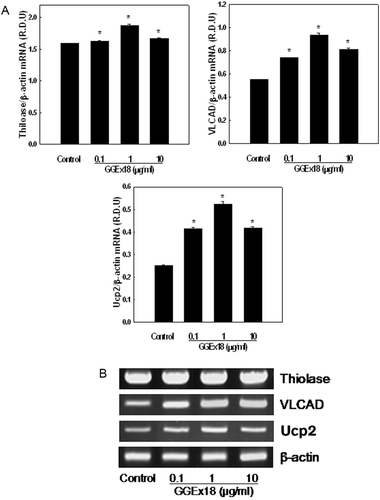
Figure 6. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα reporter gene expression in NMu2Li liver cells. NMu2Li cells were transiently transfected with pSG5-mPPARα, the reporter plasmid PPRE-TK-Luc, the β-galactosidase gene, and pBSK. Cells were treated with GGEx18 at concentrations of 0.1, 1, and 10 μg/mL or 10 μM fenofibrate (FF). After incubation for 24 h, cells were harvested and subsequently assayed for luciferase and β-galactosidase activities. Data are expressed as the mean ± standard deviation of relative luciferase units/β-galactosidase activity for three experiments. *p < 0.05 compared with the control group.
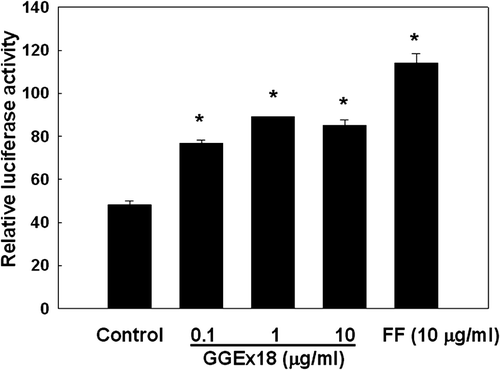
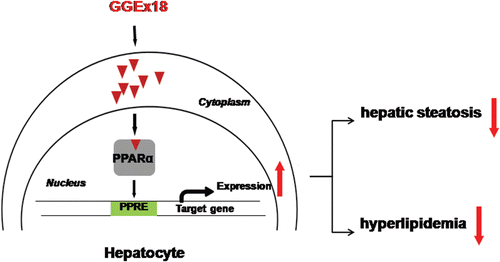
Figure 7. Possible mechanism of Gyeongshingangjeehwan 18 (GGEx18) to inhibit hepatic steatosis and hyperlipidemia. GGEx18 may activate PPARα target genes responsible for fatty acid oxidation in the livers of obese mice, leading to decreased hepatic steatosis, hypertriglyceridemia, and hypercholesterolemia.