Figures & data
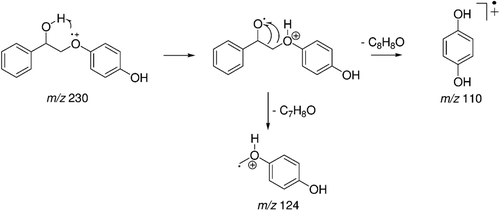
Figure 1. Reduced glutathione (GSH) concentrations (µmol/mg of protein) on rat hepatocytes after treatment with 2-phenoxy-1-phenylethanone (LS-2) from 0 to 1 mM for 24 and 48 h. Zero concentration refers to untreated hepatocytes that received only DMEM culture medium containing 1% DMSO. Mean ± SEM for six and two independent experiments for 24 and 48 h, respectively.
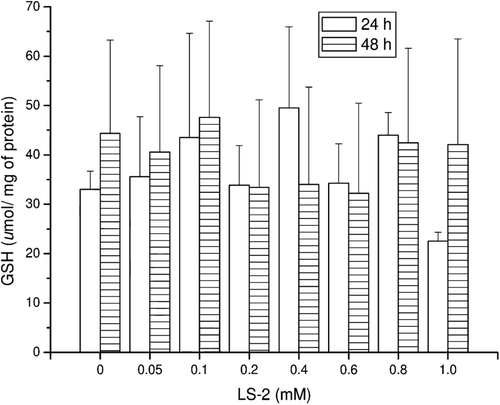
Figure 2. TBARS concentrations (nmol/mg of protein) in rat hepatocytes after treatment with 2-phenoxy-1-phenylethanone (LS-2) from 0 to 1 mM for 24 and 48 h. Zero refers to cells incubated with DMEM containing 1% DMSO as control. Mean ± SEM for six and two independent experiments for 24 and 48 h, respectively.
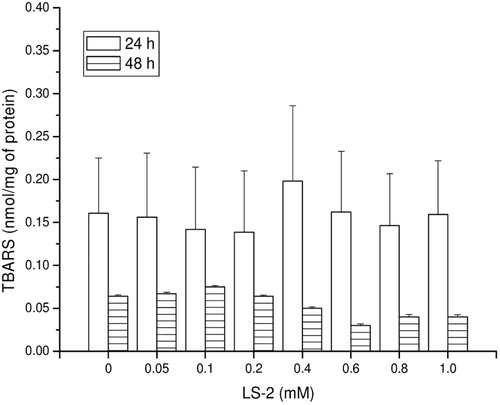
Figure 3. 7-methoxyresorufin dealkylase (CYP4501A2) activity in rat hepatocytes after treatment with 2-phenoxy-1-phenylethanone (LS-2) from 0 to 1 mM for 24 h. Results are expressed as amount of 7-resorufin formed per milligram of cell lysate protein (µmol/mg of protein). Mean ± SEM for six independent experiments.
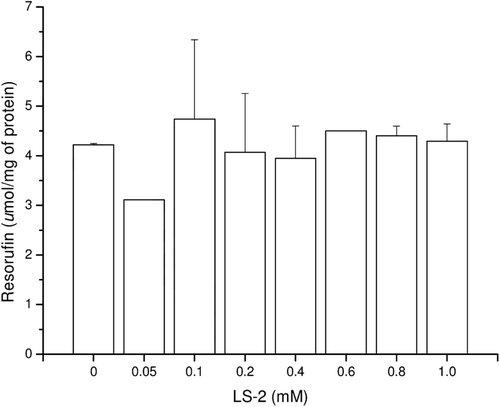
Figure 4. Coumarin 7-hydroxylase activity (CYP2A1 and CYP2A2) in rat hepatocytes after treatment with 2-phenoxy-1-phenylethanone (LS-2) from 0 to 1 mM for 48 h. Results expressed as 7-hydroxycoumarin formed per milligram of cell lysate protein (µmol/mg of protein). Mean ± SEM for two independent experiments.
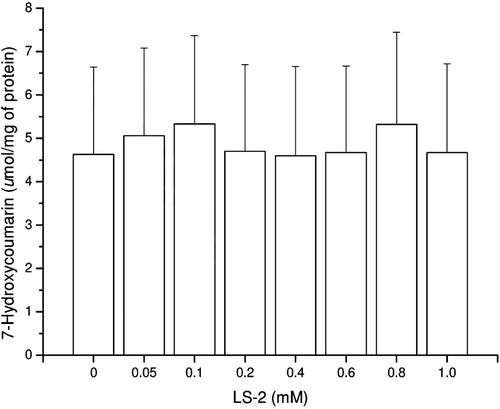
Figure 5. GC-MS profile for 2-phenoxy-1-phenylethanone (LS-2) and metabolites from the hepatocytes treated with LS-2 for 24 h. Peaks one, two, and four represent the LS-2 molecule and metabolites 1 and 2, respectively. Peaks three and five are phthalates; Peak * is an unidentified compound.
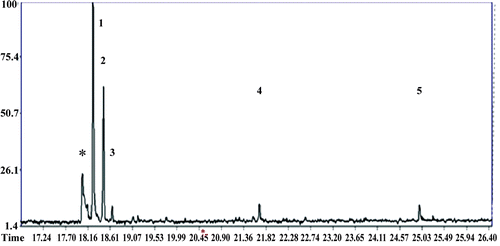
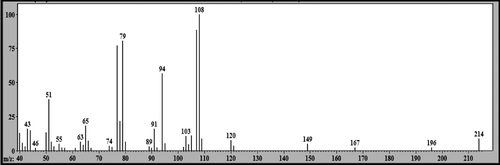
Figure 6. Mass spectrum (EI): peak with retention time (Rt) = 18.25 min attributed to 2-phenoxy-1-phenylethanone (LS-2).
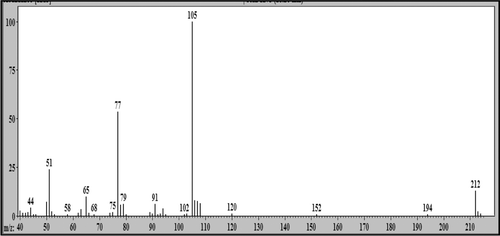
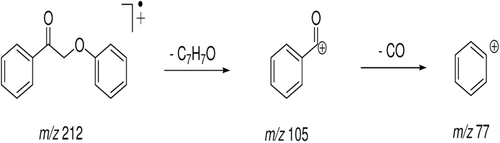
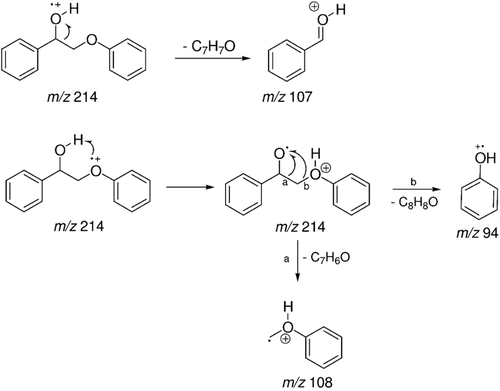
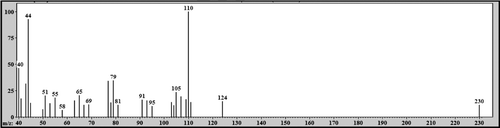
Figure 8. Mass spectrum (EI) of the peak with retention time (Rt) = 18.47 min attributed to 2-phenoxy-1-phenylethanol - metabolite 1.