Figures & data
Figure 1. A – Chemical structure of 2β,3β,16α,20(R),25-pentahydroxy-22-oxocucurbita-5-en (1) isolated from Cayaponia racemosa. B – Activity of compound 1 and at 2.5 and 5.0 µg/mL on HL60 cells assessed by Trypan blue exclusion. C – Effect of cucurbitacin on the pattern of HL60 cell death, determined by acridine orange and ethidium bromide staining (AO/BE). Negative controls (C) were treated with solvent (DMSO); positive controls (+) were treated with doxorubicin (0.3 µg/mL). The cells were treated for 24 h. *p < 0.05 compared with controls by ANOVA followed by Student Newman–Keuls test.
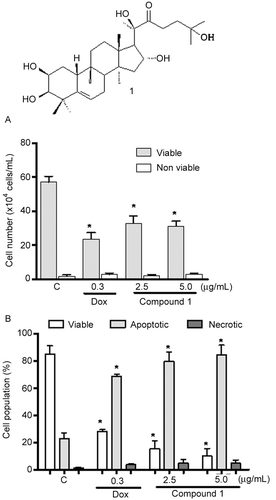
Figure 2. Cucurbitacin 1 induced morphological changes in HL-60 leukemia cells. A – Control cells; B – Cells treated with doxorubicin at 0.3 µg/mL; C and D – Cells treated with compound 1 at 2.5 and 5.0 µg/mL. Black arrows – nuclear fragments showing a periphery of compacted chromatin.
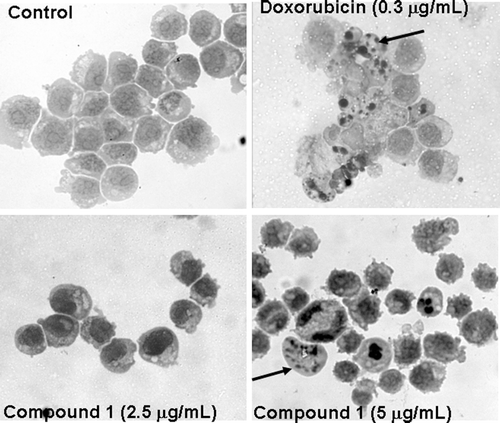
Figure 3. Tumor mass of mice transplanted with Sarcoma 180 cells after 7 days of treatment by intraperitoneal route with compound 1 (2β,3β,16α,20(R),25-pentahydroxy-22-oxocucurbita-5-en) isolated from Cayaponia racemosa and in association with 5-fluorouracil (5-FU, positive control). Negative control (C) received only DMSO 4%. Data are expressed as mean ± SEM (n = 10 animals/group). Within the square is demonstrated the tumor inhibition capacity expressed as perceptual in comparison with the negative control. *p < 0.01 compared to control by ANOVA followed by Newman–Keuls test.
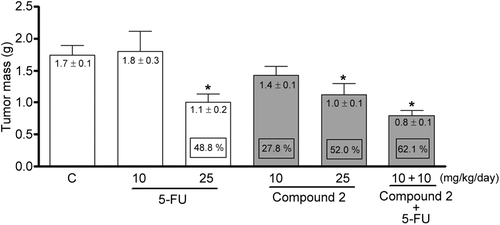
Figure 4. Histopathology of tumors excised from Sarcoma 180 transplanted mice after 7 days of intraperitoneal treatment with compound 1 (2β,3β,16α,20(R),25-pentahydroxy-22-oxocucurbita-5-en) isolated from Cayaponia racemosa. A – Negative control (DMSO 4%), B and C – 5-Fluorouracil 10 and 25 mg/kg/day, D and E – Compound 1, 10 and 25 mg/kg/day, F – 5-Fluorouracil + Compound 1 (10 + 10 mg/kg/day), respectively. Magnification, 400×.
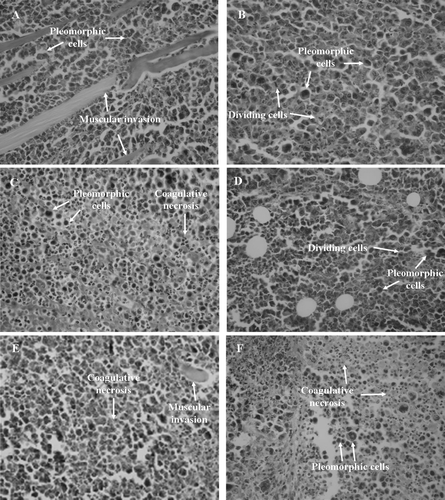
Table 1. Body effects of compound 1 (2β,3β,16α,20(R),25-pentahydroxy-22-oxocucurbita-5-en) isolated from Cayaponia racemosa on mice transplanted with Sarcoma 180 cells.
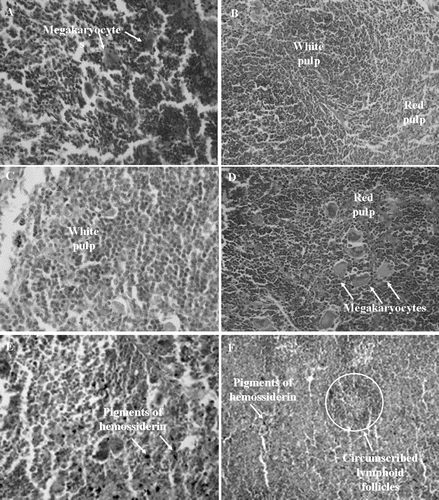
Figure 5. Histopathology of spleens excised from Sarcoma 180 transplanted mice after 7 days of intraperitoneal treatment with compound 1 (2β,3β,16α,20(R),25-pentahydroxy-22-oxocucurbita-5-en) isolated from Cayaponia racemosa. A – Negative control (DMSO 4%), B and C – 5-Fluorouracil 10 and 25 mg/kg/day, D and E – Compound 1, 10 and 25 mg/kg/day, F – 5-Fluorouracil + Compound 1 (10 + 10 mg/kg/day), respectively. Magnification, 400×.