Figures & data
Figure 1. Kugelrohr distillation apparatus. A – oven (350 W); B – sample flask; C – receipting flask; D – cooling bath (dry ice/acetone); E – rotary system/vacuum outlet/support.
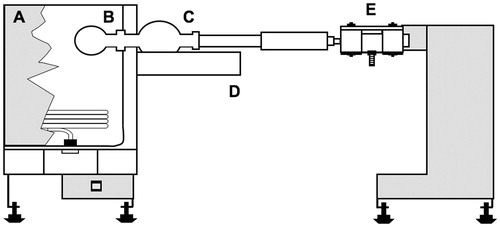
Table 1. Constitution of the leaf essential oils and the more (+V) and less (−V) volatile fractions from S. cumini and P. guajava after the Kugelrhor distillation.
Figure 2. Relative contents of monoterpenes and sesquiterpenes in the original samples and the more (+V) and less (−V) volatile fractions from distillation of S. cumini (SC) and P. guajava (PG) essential oils. Identified compounds: M-Hc = monoterpenes hydrocarbons; M-Ox = oxydized monoterpenes; S-Hc = sesquiterpenes hydrocarbons; S-Ox = oxydized sesquiterpenes. Identified compounds: M-Hc = monoterpenes hydrocarbons; M-Ox = oxydized monoterpenes; S-Hc = sesquiterpenes hydrocarbons; S-Ox = oxydized sesquiterpenes.
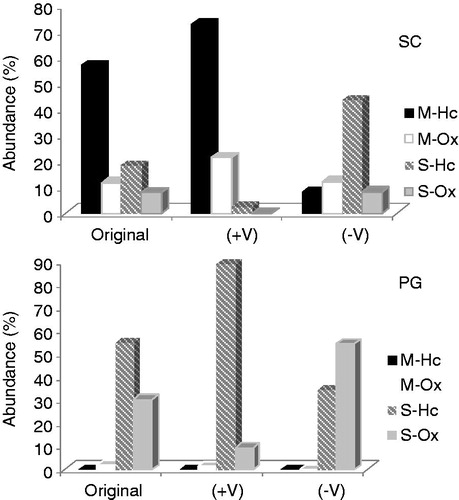
Figure 3. Effect of S. cumini (SC), more (+V) and less (−V) volatile fractions on LPS-induced total leukocyte, mononuclear cells, neutrophils and eosinophils recruitment. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively.
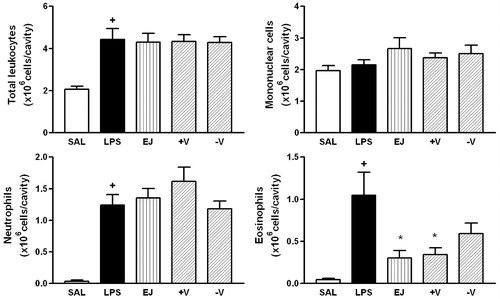
Figure 4. Effect of P. guajava (PG), more (+V) and less (−V) volatile fractions on LPS-induced total leukocyte, mononuclear cells, neutrophils and eosinophils recruitment. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively.
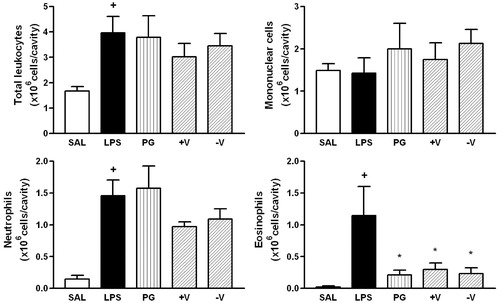
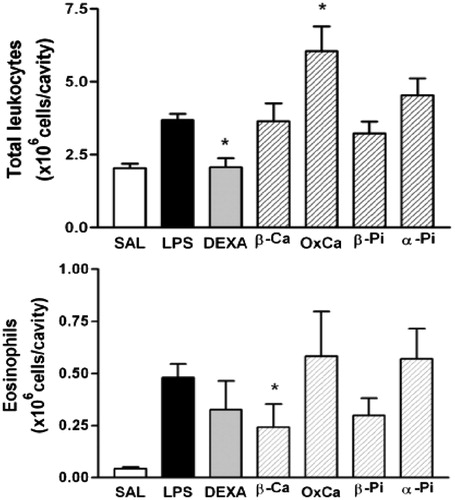
Figure 5. Effect of β-caryophyllene (β-Ca), β-caryophyllene oxide (OxCa), β-pinene (β-Pi) and α-pinene (α-Pi) on LPS-induced total leukocyte and eosinophils recruitment. Sample (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. * Indicates p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively. Dexametasona (DEXA, 2 mg/kg) was the positive control.