Figures & data
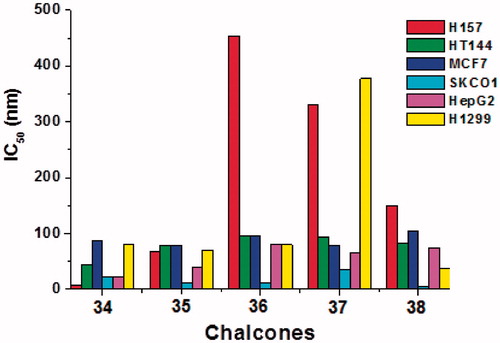
Table 1. The brine shrimp cytotoxicity of the synthesized chalcones.  .
.
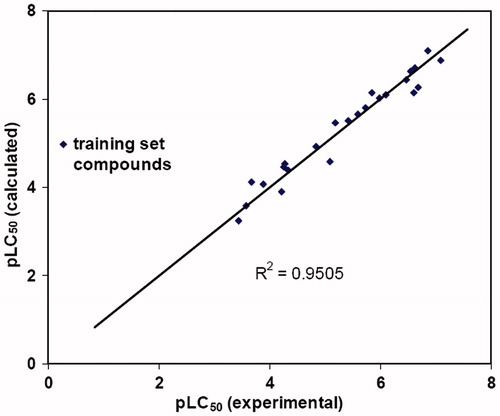
Table 2. Observed and predicted pLC50 of the training set compounds.
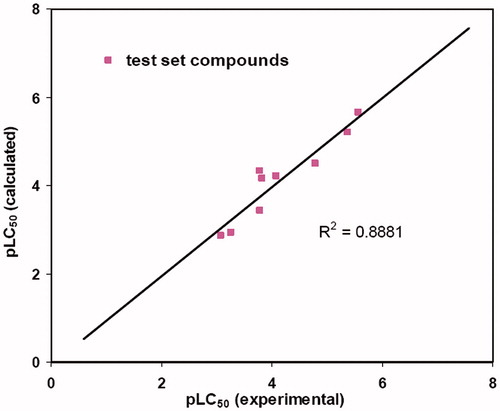
Table 3. Observed and predicted pLC50 of the test set compounds.
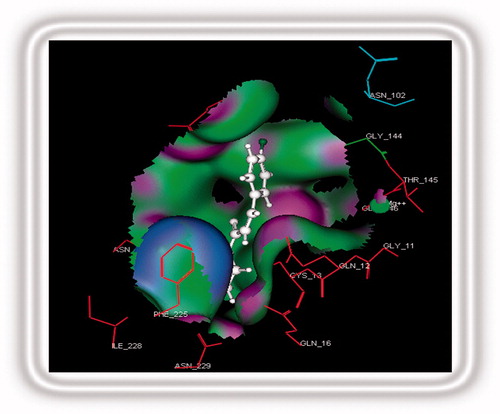
Figure 4. Stereoview of the CoMFA map for the electronic and steric contributions favoring activity.
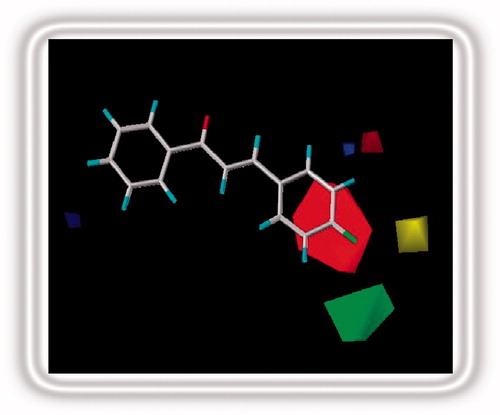
Table 4. Predicted pLC50 of the designed chalcones.
Table 5. The energy scores (kCal mol−1) of designed inhibitors.
Table 6. The predicted and observed pLC50 values of the designed chalcone analogues.