Figures & data
Figure 1. Maysin and its derivatives found in centipedegrass. (A) Overflow of the cascade for extraction and purification of maysin from CG. CGE, centipedegrass extract; EA-CG, ethyl acetate extract. (B) Compound contents (µg/g extract) of maysin and its derivatives in the centipedegrass fraction (EA-CG). The values are presented as the means ± SD (n = 3) and are given as mg/L of the investigated bitter gourd fractions.
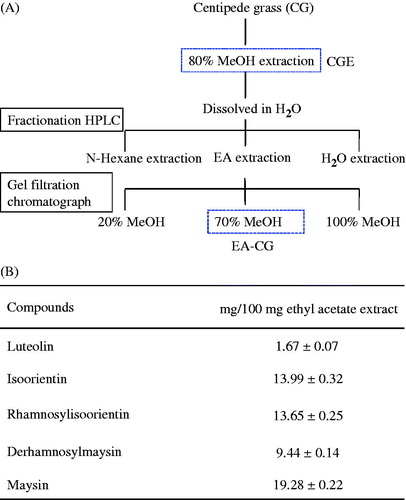
Figure 2. EA-CG inhibited Aβ oligomerization. (A) Chemical structure of maysin. (B) Oligomeric Aβ species were prepared by incubating Aβ42 and Aβ oligomers with or without EA-CG compounds. The oligomers species, including monomer, oligomer, and highly aggregated Aβ, were separated following electrophoresis by 12% Tris-Tricine SDS-PAGE. The representative Western blot from one of the three independent experiments is shown.
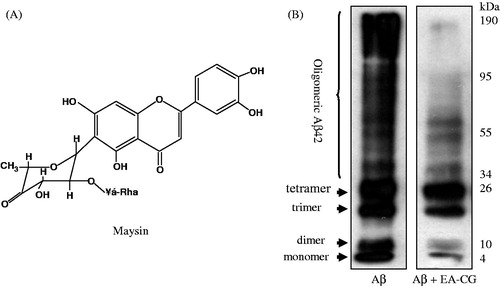
Figure 3. EA-CG dose-dependently inhibited BACE1 activity. The BACE1 enzyme activity was determined by incubating recombinant human BACE1 and its substrate, Rh-EVNLDAEFK, which was also used as a quencher in the presence or in the absence of CGE or EA-CG. The fluorescence activity was analyzed by dose response with CGE or EA-CG, and resveratrol was used as the positive control. Comparison of BACE1 inhibition was performed, and the results were described as the inhibition rate (% of control). Representative results are based on three independent experiments, and the values are presented as the means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with 5 µg/mL CGs-treated cells.
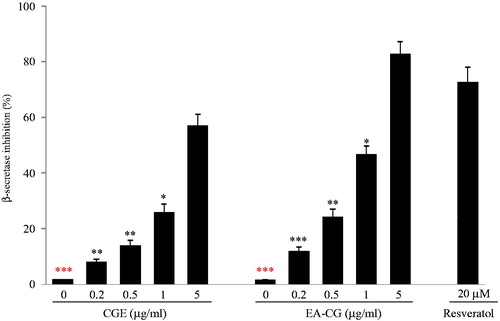
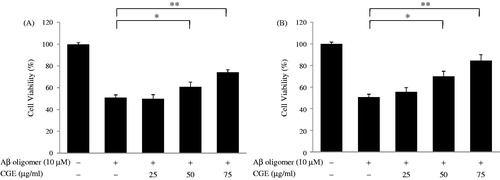
Figure 4. Maysin and its derivatives inhibited Aβ-mediated neuronal cell toxicity in a dose-dependent manner. Neuronal PC12 cells were cultured with Aβ oligomer (10 µM) for 24 h in the presence of various doses of CGE or EA-CG, and cell toxicity was determined using the MTT assay. These data were presented as relative cell viability values. (A) The effect of CGE on Aβ-mediated neuronal cell toxicity. Representative results are expressed as the means ± SD following three independent experiments. *p < 0.05, significantly different from the Aβ oligomer-treated group; **p < 0.01, significantly different from the Aβ oligomer-treated group. (B) The effect of EA-CG on Aβ-mediated, neuronal cell toxicity. Representative results are expressed as the means ± SD following three independent experiments. *p < 0.05, **p < 0.01.