Figures & data
Table 1. Primer sequences for TNF-α, IL-1β, IL-6, IL-8, NF-κB, smad-3, smad-7, VEGF, pol-γ, TGF-β, Bax, collagen-1, and β-actin.
Table 2. Physicochemical evaluations of different formulations of naringin ointment.
Figure 2. Effect of naringin ointment treatment (1, 2, and 4%) on wound area (mm2) (A) and rate of wound contraction (%) (B) in rats. Data are expressed as mean ± SEM and analyzed by two-way analysis of variance followed by Bonferroni's test. *p < 0.05 as compared with the vehicle control group and $p < 0.05 as compared with one another. VC, vehicle control; FSC (1%), framycine sulfate ointment (1% w/w)-treated group; N (1%), naringin ointment (1%, w/w)-treated group; N (2%), naringin ointment (2%, w/w)-treated group; N (4%), naringin ointment (4%, w/w)-treated group.
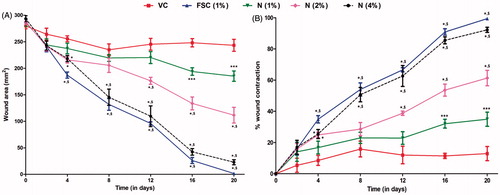
Table 3. Effect of naringin ointment treatment on the period of epithelization, wound index, scar width, CT-50, tensile strength, hydroxyproline content and protein content of rats.
Table 4. Effect of naringin ointment treatment on SOD, GSH, MDA, MPO, and NO levels in wound tissue of rats.
Figure 3. Effect of naringin ointment treatment (1, 2, and 4%) on mRNA expression of TNF-α, IL-1β, IL-6, IL-8, NF-κB, and pol-γ in wound tissue as determined by relative quantification by reverse transcriptase polymerase chain reaction analysis (A), quantitative representation of mRNA expression of TNF-α (B), IL-1β (C), IL-6 (D), IL-8 (E), NF-κB (F), and Pol-γ (G). Data are expressed as mean ± SEM and analyzed by one-way analysis of variance followed by Tukey's multiple range test. #p < 0.05 as compared with the normal group; *p < 0.05 as compared with the vehicle control group; $p < 0.05 as compared with one another. VC, vehicle control; FSC (1%), framycine sulfate ointment (1%, w/w)-treated group; N (1%), naringin ointment (1%, w/w)-treated group; N (2%), naringin ointment (2% w/w)-treated group; N (4%), naringin ointment (4%, w/w)-treated group.
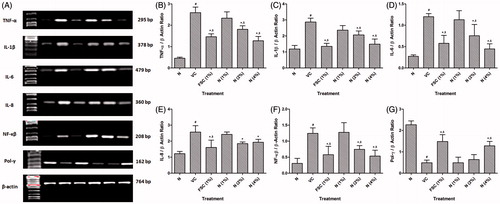
Figure 4. Effect of naringin ointment treatment (1, 2, and 4%) on mRNA expression of smad-3, smad-7, TGF-β, VEGF, Bax, and collagen-1 in wound tissue as determined by relative quantification by reverse transcriptase polymerase chain reaction analysis (A), quantitative representation of mRNA expression of smad-3 (B), smad-7 (C), TGF-β (D), VEGF (E), Bax (F), and collagen-1 (G). Data are expressed as mean ± SEM and analyzed by one-way analysis of variance followed by Tukey's multiple range test. #p < 0.05 as compared with the normal group; *p < 0.05 as compared with the vehicle control group; $p < 0.05 as compared with one another. VC, vehicle control; FSC (1%), framycine sulfate ointment (1% w/w)-treated group; N (1%), naringin ointment (1% w/w)-treated group; N (2%), naringin ointment (2%, w/w)-treated group; N (4%), naringin ointment (4% w/w)-treated group.
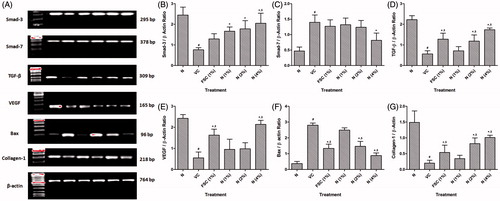
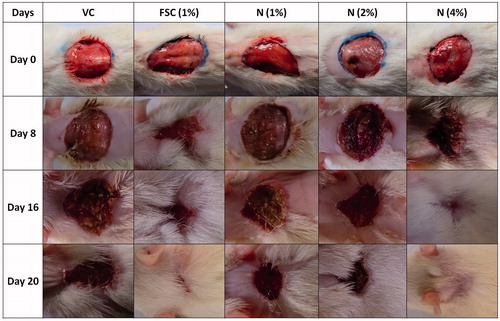
Figure 5. Photomicrographs of sections of hematoxylin and eosin-stained wound skin tissues. Microscopic image of wound skin of normal rat (A), vehicle control group (B), framycine sulfate ointment (1% w/w)-treated group (C), naringin ointment (1%, w/w)-treated group (D), naringin ointment (2%, w/w)-treated group (E); naringin ointment (4%, w/w)-treated group. Images (×100 magnification) are typical and are representative of each study group.
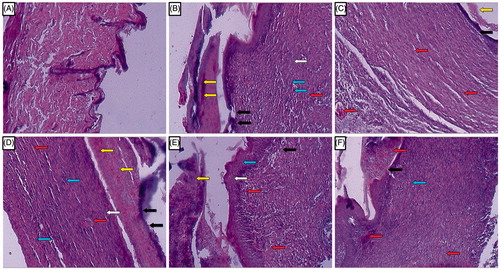
Table 5. Effect of naringin ointment treatment on wound healing processes and healing phases of rats.
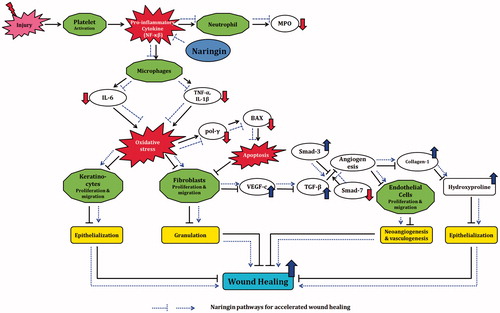
Figure 6. Possible molecular mechanisms through which naringin ointment may improve wound healing. In the cascade of a normal wound healing process, activation of platelets after injury causes release of pro-inflammatory cytokines like TNF-α and IL-1β that activate macrophages. Further release of TNF-α, IL-1β, and IL-6 results in inhibition of fibroblast as well as keratinocytes proliferation and migration, which delays epithelization and granulation. Inhibition of fibroblast proliferation results in decreased VEGF-c synthesis and thus in down-regulation of TGF-β1 expression. This cascade leads to decreased endothelial cell proliferation and thus to delayed neoangiogenesis and vasculogenesis. Decreased collagen synthesis results in decreased epithelization. Together, these phenomena cause delayed wound healing. Naringin ointment reduces the elevated oxidative stress and modulates the expression of growth factors (VEGF-c and TGF-β1), inflammatory mediators (TNF-α, IL-1β, and IL-6), and apoptotic mediators (pol-γ and BAX) thereby up-regulated collagen-1 expression, which improves the wound healing process (black line reflects the delayed wound healing process whereas blue lines indicate the proposed mechanism of naringin in wound healing).