Figures & data
Table 1. Effects of long-term carnosine administration on the final body weight (BW), liver weight (LW), serum ALT, AST, ALP, LDH, albumin and total protein (TP) in different animal groups at the end of experiments (n = 7).
Figure 1. Effects of long-term carnosine administration on lipid peroxidation measured as hepatic malondialdehyde (MDA) content in control, lead, carnosine (10 mg/kg) treated control (Carnosine) and carnosine (10 mg/kg) treated lead (lead + carnosine) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± S.E.M. **p < 0.01 and ***p < 0.001 (as compared with the control group).
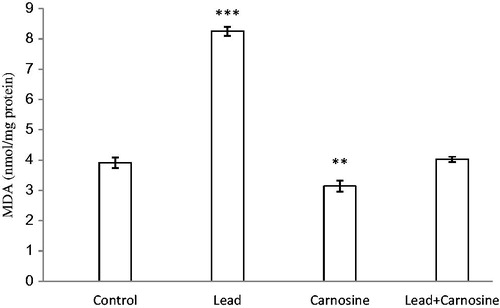
Figure 2. Effects of long-term carnosine administration on total thiol concentration in liver homogenates samples of control, lead, carnosine (10 mg/kg) treated control (carnosine) and carnosine (10 mg/kg) treated lead (lead + carnosine) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± S.E.M. *p < 0.05, **p < 0.01 and ***p < 0.001 (as compared with the control group).
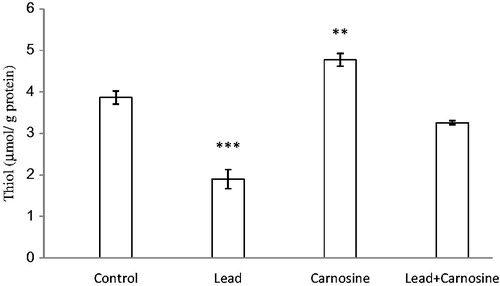
Figure 3. Effects of long-term carnosine administration on antioxidant power (FRAP value) in liver homogenates samples of control, lead, carnosine (10 mg/kg) treated control (carnosine) and carnosine (10 mg/kg) treated lead (lead + carnosine) groups (n =7) at 8 weeks after treatments. The data are represented as mean ± SEM. *p<0.05, **p<0.01 and * * *p<0.001 (as compared with the control group).
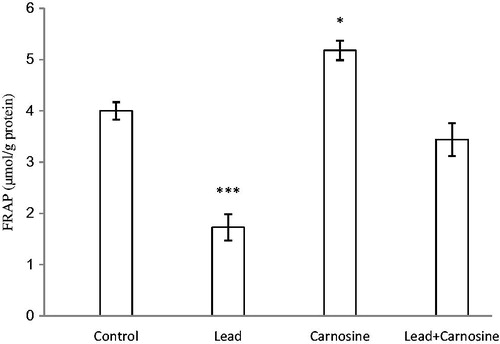
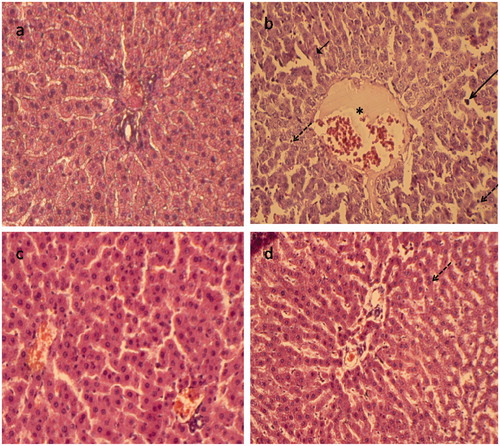
Figure 4. Paraffin sections stained by haematoxylin and eosin (H&E) for histopathological examination of liver tissues of rats as follows: (a) the control rats; (b) rats treated with lead (500 mg Pb/L in the drinking water); (c) rats fed with carnosine (10 mg/kg, i.g.) and (d) rats treated with lead (500 mg Pb/L in the drinking water) and fed with carnosine (10 mg/kg, i.g.). The long arrow indicates infiltrating leukocyte. The short arrow indicates pyknotic nucleus. The dashed arrow indicates hepatic cell necrosis. The asterisk indicates the distended portal vein.