Figures & data
Figure 1. Chemical structures and product ion spectra of harmane (a); harmine (b); IS (c); M1–M3 (d); M4 (e); M5 (f); M6, M7 and M9 (g); M19 and M20 (h).
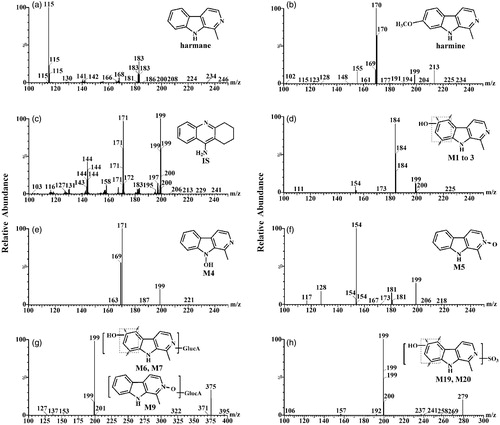
Table 1. The optimized MS analytical parameters of harmane, its 10 metabolites, harmine and IS.
Figure 2. Representative MRM chromatograms of blank rat plasma sample (a); blank plasma spiked with harmane and harmine at LLOQ and IS (b); and plasma sample spiked with IS after oral administration of harmane at a dose of 30.0 mg/kg (c).
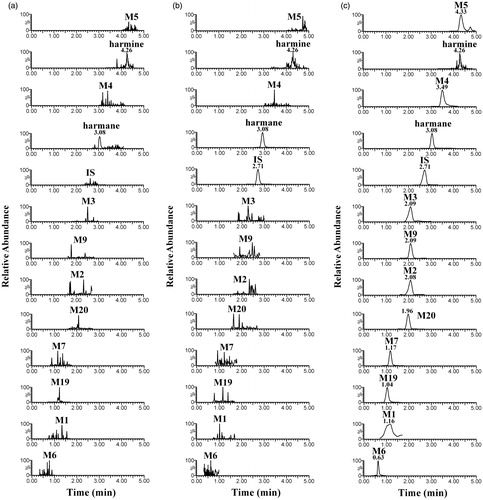
Table 2. Accuracy, precision, extraction recovery, matrix effect, and stability of harmane, harmine and IS in rat plasma (n = 5).
Figure 3. Mean plasma concentration–time curves of harmane in rats after intravenous and oral administration at doses of 1.0 and 30.0 mg/kg, respectively.
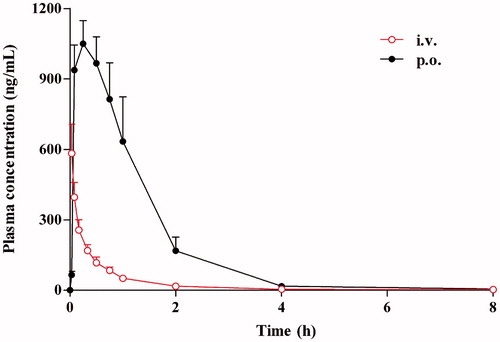
Figure 4. Mean plasma concentration–time curves of phase I metabolites after intravenous administration of harmane at a dose of 1.0 mg/kg (a); phase I metabolites after oral administration of harmane at a dose of 30.0 mg/kg (b); phase II metabolites after intravenous administration of harmane at a dose of 1.0 mg/kg (c); and phase II metabolites after oral administration of harmane at a dose of 30.0 mg/kg (d).
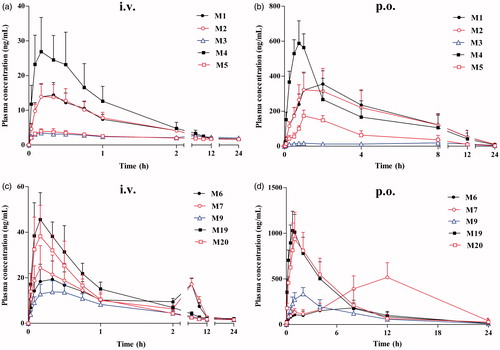
Table 3. Pharmacokinetic parameters of harmane and its 10 metabolites (M1–M7, M9, M19 and M20) in rats after intravenous administration of harmane at a dose of 1.0 mg/kg (mean ± SD, n = 8).
Table 4. Pharmacokinetic parameters of harmane and its 10 metabolites (M1–M7, M9, M19 and M20) in rats after oral administration of harmane at a dose of 30.0 mg/kg (mean ± SD, n = 8).
Figure 5. Comparison of the absolute bioavailability of harmane and its 10 metabolites (M1–M7, M9, M19 and M20) in rats after oral administration of harmane at a dose of 30.0 mg/kg.
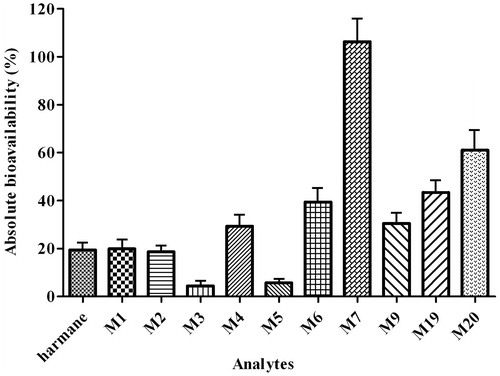
Figure 6. Mean plasma concentration–time curves of harmane (a) and harmine (b) in normal and test rats after intravenous and oral administration of harmane.
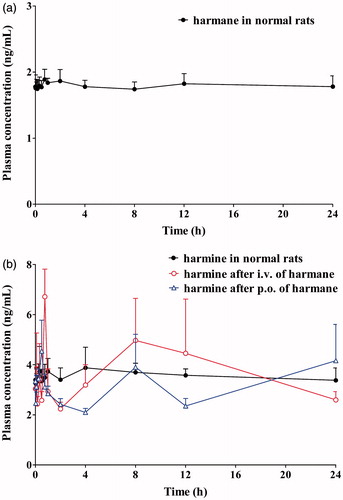
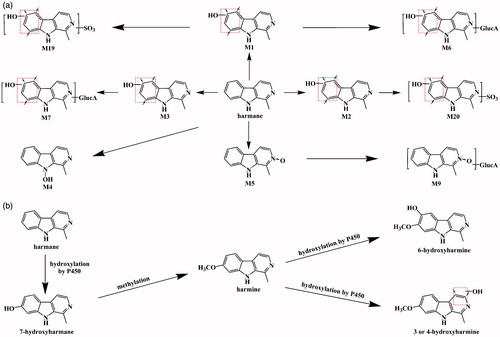
Figure 7. Proposed metabolic pathways of harmane: the present proposed metabolic pathways of harmane in rats after intravenous and oral administration of harmane at doses of 1.0 and 30.0 mg/kg, respectively (a); the published proposed metabolic pathways for harmane and harmine (b) (Guan et al. Citation2001).