Figures & data
Figure 1. Flow chart for the extraction, fractionation and compounds isolation from Hamelia patens. The leaves were subjected to sequential maceration with hexane, dichloromethane and methanol. Chlorophyll was removed with activated carbon or by filtration over celite. The hexane extract (HEX) without chlorophyll was fractionated by silica–gel column chromatography. Fractions 1 and 12 were purified by preparative plate chromatography, yielding two compounds. The dichloromethane and methanol extracts were subjected to liquid–liquid extraction with ethyl acetate (DCM–EtOAc and MeOH–EtOAc). The residue of the aqueous phase of the methanol extract was extracted again with methanol (MeOH–Aq).
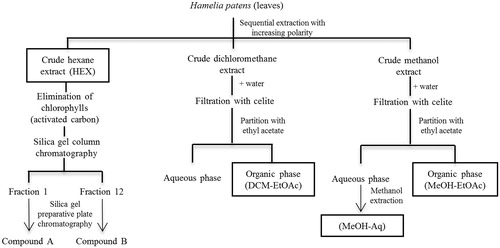
Figure 2. Effect of the HEX extract and its fractions from Hamelia patens on carrageenan-induced paw oedema. The oedema volume was measured in rats treated orally with: (A) vehicle, HEX-50, HEX-200 and HEX-500 (HEX extracts at 50, 200 and 500 mg/kg, respectively), indomethacin (7.5 mg/kg) and (B) fractions 1 and 12 obtained from the HEX extract. Values represent the mean ± SEM, n = 6, *p < 0.05, **p < 0.01, ***p < 0.001 significantly different compared with the vehicle control group (two-way ANOVA followed by Bonferroni’s post-test).
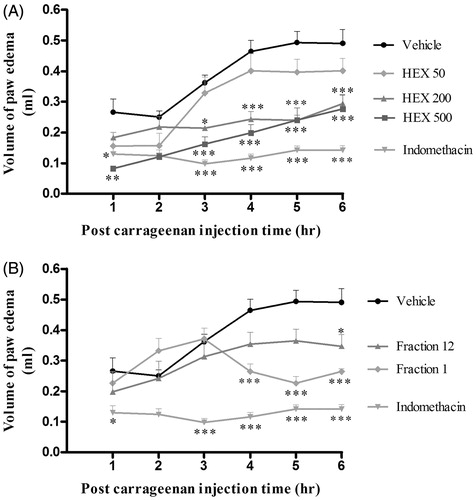
Figure 3. Topical effect of the extracts from Hamelia patens at 1 and 0.5 mg/ear on (A) TPA-induced ear oedema (weight of biopsy) measured at 4 h and (B) percentage inhibition of myeloperoxidase. Hexane extract (HEX), organic phase of the dichloromethane extract (DCM–EtOAC), methanol extraction of the aqueous phase of the crude methanol extract (MeOH–Aq) and organic phase of the methanol extract (MeOH–EtOAc). Values represent the mean ± SEM, n = 5, **p < 0.01, ***p < 0.001 significantly different compared with control (TPA + vehicle), #p < 0.001 significantly different compared with the group at 1 mg/ear (one-way ANOVA followed by Tukey’s post-test).
Figure 4. Chemical structure of (6E,10E,14E,18E)-2,6,10,14,18,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene isolated from the HEX extract of Hamelia patens.
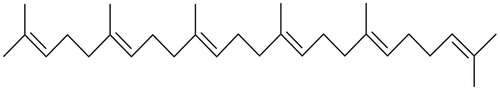
Table 1. Topical effect of β-sitosterol and stigmasterol on TPA-induced ear oedema and myeloperoxidase inhibition.
Table 2. DPPH radical scavenging activity and α-glucosidase inhibition of extracts and fractions from H. patens.
