Figures & data
Figure 1. (A) KBM-5 cells were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 1 nM TNF for 24 h. Cell viability was then analyzed by MTT assay. (B) K562 cells were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 1 nM TNF for 24 h. Cell viability was then analyzed by the MTT assay. (C) KBM-5 cells (1 × 106 cells/ml) were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 1 nM TNF for 24 h. Cell death was determined by the calcein-AM based live/dead assay, as described in “Live/dead cell assay” section. (D) Cells were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 1 nM TNF for 24 h. after which the cells were washed, fixed, stained with PI and analyzed for DNA content by flow cytometry. (E) Cells were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 1 nM TNF for 24 h. Cells were incubated with FITC-conjugated antibody to annexin V, and then analyzed by flow cytometry for early apoptotic effects.
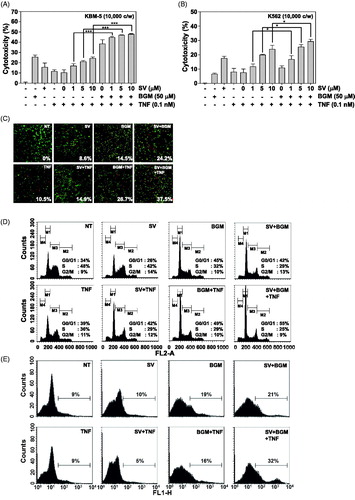
Figure 2. (A) KBM-5 cells were treated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Whole-cell extracts were prepared, separated on SDS-PAGE and subjected to Western blotting using antibodies against the cIAP-1, Bcl-2, Bcl-xL and Survivin. (B) KBM-5 cells were treated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Whole-cell extracts were prepared, separated on SDS-PAGE and subjected to Western blotting using antibodies against the Cyclin D1 and MMP-9. (C) KBM-5 cells were treated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Whole-cell extracts were prepared, separated on SDS-PAGE and subjected to Western blotting using antibodies against the p53, p27 and p21. β-Actin was used as a loading control. (D) KBM-5 cells pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Whole-cell extract were prepared and analyzed by Western blotting using the caspase-3 antibody. (E) KBM-5 cells were pretreated 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. PARP cleavage was determined by Western blot analysis. β-Actin was used as a loading control. (F) KBM-5 cells pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 30 min. Whole-cell extract were prepared and analyzed by Western blotting using the p-STAT3(Tyr705) and STAT3 antibody.
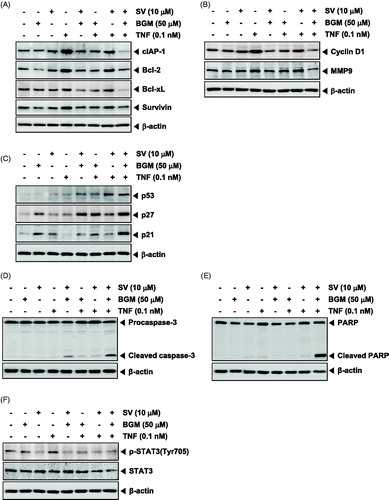
Figure 3. (A) Nuclear extracts were prepared from untreated KBM-5 cells or cells pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, stimulated 0.1 nM TNF for 30 min, and then assayed for NF-κB binding to DNA by EMSA. (B) Cytoplasmic extracts were prepared from untreated KBM-5 cells or cells pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 30 min, and then subjected to Western blotting using antibody against the IκBα. β-Actin was used as a loading control. (C) Nuclear extracts were prepared from untreated KBM-5 cells or cells pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, stimulated 0.1 nM TNF for 30 min, and then subjected to Western blotting using antibody against the p65. Lamin B was used as a loading control.
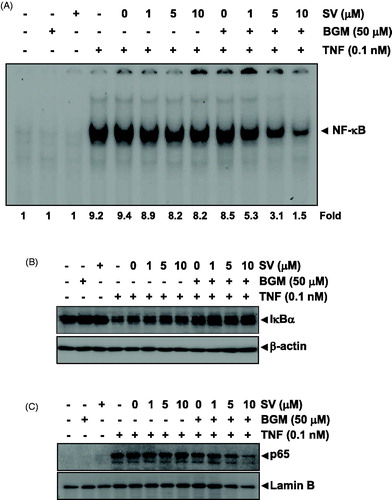
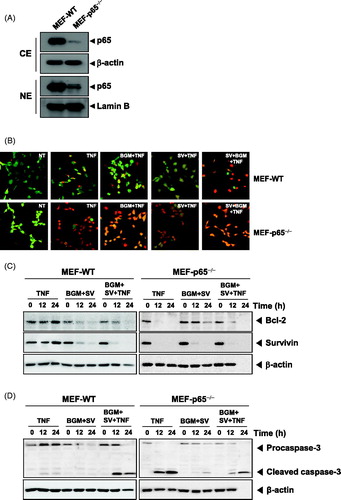
Figure 4. (A) Cytoplasmic and nuclear extracts were prepared from MEF wild-type and MEF p65−/− cells, separated on SDS-PAGE and electrotransferred to a nitrocellulose membrane. The analysis was performed using p65 antibody. (B) The MEF wild-type and MEF p65−/− (1 × 105 cells/ml) cells were pretreated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Cells were stained with live/dead assay reagent for 30 min and then analyzed under a fluorescence microscope. (C) MEF wild-type and MEF p65−/− (1 × 106 cells/ml) cells were treated with 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Whole-cell extracts were prepared, separated on SDS-PAGE and subjected to Western blotting using antibodies against the Bcl-2 and Survivin. β-Actin was used as a loading control. (F) MEF wild-type and MEF p65−/− (1 × 106 cells/ml) cells were pretreated 10 μM SV in the presence or absence of 50 μM BGM for 12 h, incubated 0.1 nM TNF for 24 h. Caspase-3 cleavage was determined by Western blot analysis. β-actin was used as a loading control.