Figures & data
Table 1. List of antibodies along with other details.
Figure 1. Trypan blue dye exclusion assay of isolated rat islets: (a) Isolated islets and DTZ staining. (b) Trypan blue dye exclusion assay to identify the pretreatment dose and time of incubation for EL MeOH ext. in a time dependent and dose dependent study assessing its maximum protective effect against 50 μM H2O2 induced cell death (n = 3).
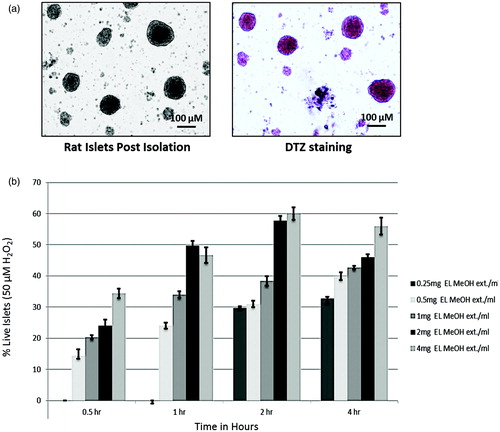
Figure 2. FDA/PI and DCFDA Staining: (a) Qualitative assessment of islet viability when exposed to 50 μM H2O2, when pre-incubated with EL extract compared to the control group. (b) Comparative fluorescent intensity plot for FDA/PI staining (***p ≤ 0.001 versus Control FDA; @@p ≤ 0.01 versus Control PI; $p ≤ 0.05 versus H2O2 treated FDA; #p ≤ 0.05 versus H2O2-treated PI). DCFDA staining representing the amount of ROS generation. (c) Comparative qualitative assessment of the DCFDA staining between the control, 50 μM H2O2 and the EL extract-treated groups. Here, the DCFDA gives green fluorescence in the presence of ROS in direct proportion. Hence, this figure indicates the presence of ROS, which is higher in the H2O2-treated group compared to EL treated and (d) Quantitative graphical representation for the same (***p ≤ 0.001 versus Control group; $$$p ≤ 0.001 versus H2O2-treated group) (n = 3).
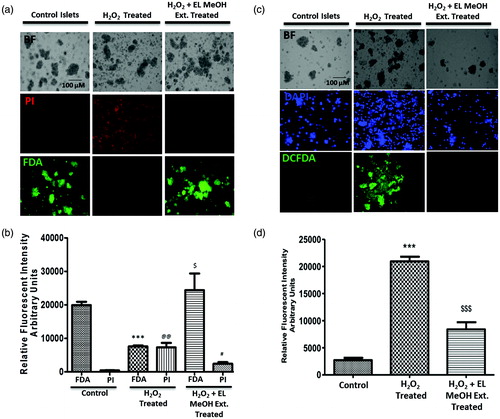
Figure 3. Cell death determined by Annexin V-FITC (PS)/PI staining and DNA damage by comet assay: (a) H2O2-treated group demonstrated significant increase in staining for PS and PI from the control group indicating cell death. However, there was no difference in staining of PS and PI in the EL pre-treated group and the control group but a significant reduction in stain uptake between the H2O2-treated and EL pre-treated group, which suggested that pre-incubation with EL protects the islets from undergoing oxidative stress induced cell death, which was quantified and depicted (b) (**p ≤ 0.01 versus Control Annexin V; @@p ≤ 0.01 versus Control PI; $$p ≤ 0.01 versus H2O2 treated Annexin V; ##p ≤ 0.01 versus H2O2-treated PI). (c) Comet Assay where the tailing is more pronounced in the case of H2O2 treated group suggesting DNA fragmentation, whereas the tailing is not seen in the case of EL-treated group giving a direct evidence of effective protection against DNA damage (n = 3).
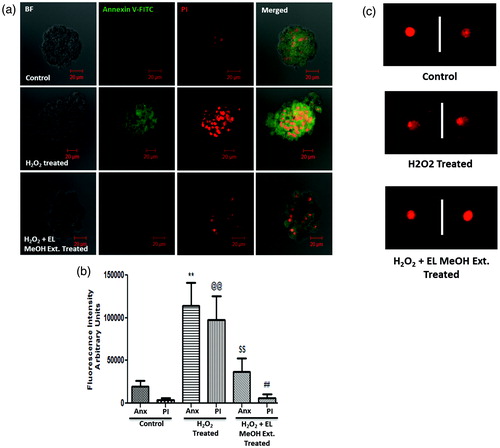
Figure 4. Immunoblotting assay: (a) Western blot analysis for the following proteins to evaluate the protective effect of EL extract against 50 μM H2O2 induced apoptosis, Caspase-3, PARP-1, TNF-α, MafA, P-P38 MAPK and β-actin as endogenous control. Further, the protein expression profile is analyzed densitometrically. (b, c, e and f) Densitometry plots of activated caspase-3, cleaved PARP-1, phospho p-38 MAPK and TNF-α respectively where EL pretreatment significantly decrease there expression level after H2O2 treatment back to that of the control expression levels. (d) Densitometry plot for MafA which remain unchanged as discussed above (* indicates versus Control and # indicates versus H2O2-treated group; */#p ≤ 0.05; **/##p ≤ 0.01 and ***/###p ≤ 0.001) (n = 3).
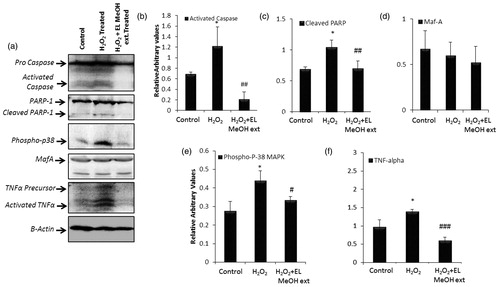
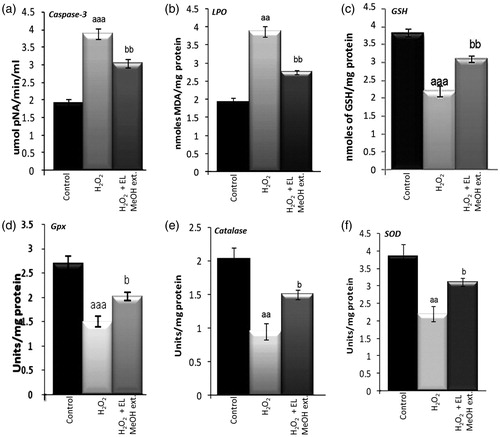
Figure 5. Effect of EL on caspase-3 activity and the antioxidant enzyme activities viz. reduced glutathione, lipid peroxidation, glutathione peroxidase, catalase and superoxide dismutase in isolated islets on 50 μM H2O2 exposure and EL MeOH ext. pretreatment: (a) Caspase-3 activity which significantly increases on exposure to H2O2 and reduces significantly when pretreated with EL. (b) Similar results with LPO is shown. (c, d, e and f) Representation of antioxidant enzyme activities of GSH, Gpx, catalase and SOD respectively. Their activities significantly decrease in response to H2O2 induced oxidative stress but gets rescued with pretreatment with EL back to control levels (a indicates versus Control and b indicates versus H2O2 treated group; a/bp ≤ 0.05; aa/bbp ≤ 0.01 and aaa/bbbp ≤ 0.001) n = 3).