Figures & data
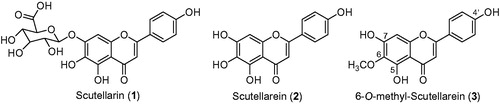
Figure 1. The chemical structures of scutellarin (1), scutellarein (2) and 6-O-methyl-scutellarein (3).
Scheme 1. Reagents and conditions: (a) HCl, EtOH, N2, reflux, 36 h, 17%; (b) Ph2CCl2 (1.5 equiv.), Ph2O, 175 °C, 30 min, 85%; (c) PhCH2Br (1.5 equiv), K2CO3 (1.75 equiv), DMF, 25 °C, 12 h, 93%; (d) HAc–H2O (4:1), reflux, 1.5 h, 95%; (e) PhCH2Br (1.3 equiv), K2CO3 (1.5 equiv), DMF, 25 °C, 12 h, 93%; (f) CH3I (1.3 equiv), K2CO3 (1.5 equiv), DMF, 25 °C, 4 h, 94%; (g) Pd/C (10 wt%), H2 (atm), THF/EtOH, 8 h, 96%.
Figure 2. UFLC/MS chromatograms of rat urine, plasma, faeces and bile samples before and after oral administration of 6-O-methyl-scutellarein (3): (a) urine sample in ESI-mode, (b) plasma sample in ESI− mode, (c) faeces sample in ESI− mode, (d) bile sample in ESI− mode.
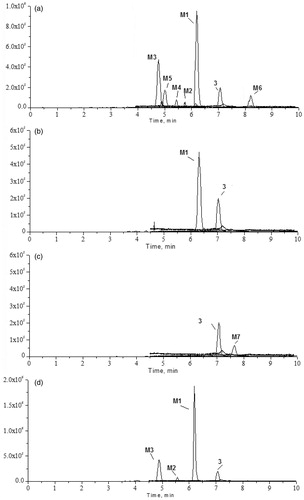
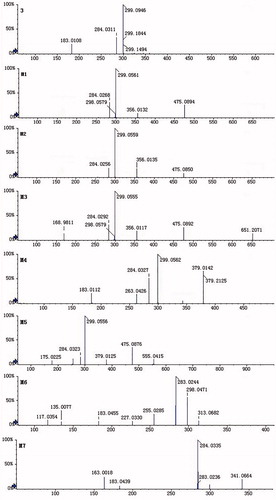
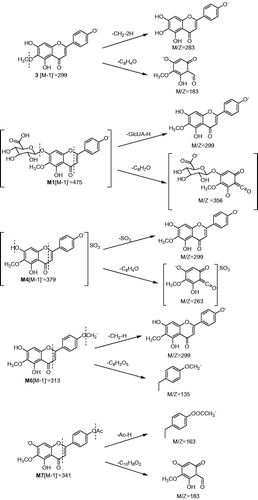
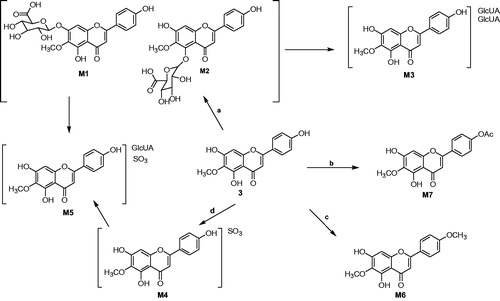
Table 1. UFLC/ESI-MS, retention time and fragment ions of 6-O-methyl-scuetellarin (3) and its metabolites in rats.