Figures & data
Figure 1. HPLC fingerprint chromatogram of Shengmai injection at 203 nm. (1) Ginsenoside Re, (2) Ginsenoside Rb1, (3) Ginsenoside Rc, (4) Ginsenoside Rb2, (5) Ginsenoside Rd and (6) Schizandrol A.
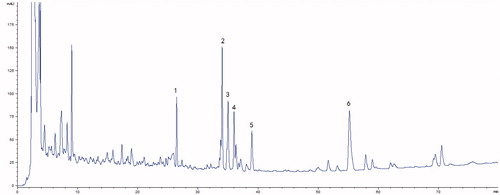
Figure 2. Effects of Shengmai injection (SMI) on the infarct volume (A, B), the neurological scores (C) and the brain water content in C57 mice subjected to tMCAO/R. Mice were subjected to 1 h of ischemia and 24 h of reperfusion (tMCAO/R). SMI (ip) was administered after 1h ischemia. 3-Methyladenine (ip) was administered at the onset of the surgery as a positive control. Images of TTC-stained brain sections (A) from Vehicle- or SMI-treated mice 24h after reperfusion. Infarct volume (B), neurological scores (C) and brain water content (D) were evaluated for Vehicle- or SMI-treated mice 24 h after reperfusion. The data are the mean ± SD, n = 6. ##p < 0.01 versus sham-operated mice, **p < 0.01 versus tMCAO/R mice.
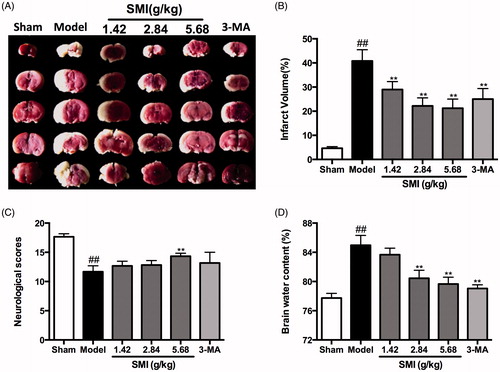
Figure 3. Effects of Shengmai injection (SMI) on the neuron’s morphological changes. Representative electron microphotographs showing neuronal injury in the ischemic penumbra of the cerebral cortex. (A) Mice subjected to the sham operation. (B) Mice subjected to 1h of ischemia and 24 h of reperfusion (tMCAO/R). (C) Mice subjected to 1 h of ischemia and 24h of reperfusion (tMCAO/R). SMI (5.68 g/kg, ip) was administered after 1h of ischemia. Scale bars = 2 μm.
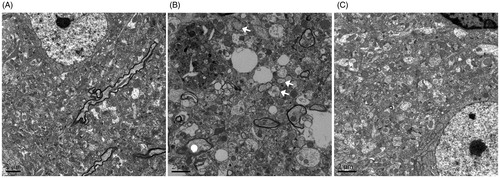
Figure 4. Effects of Shengmai injection (SMI) on the expression of LC3 in brain sections. Representative microphotographs showing immunofluorescence staining for LC3. (A) Mice subjected to the sham operation. (B) Mice subjected to 1h of ischemia and 24h of reperfusion (tMCAO/R). (C) Mice subjected to 1h of ischemia and 24 h of reperfusion (tMCAO/R). SMI (5.68 g/kg, ip) was administered after 1 h of ischemia. Representative brain sections from the ischemic penumbra of the cortex are shown. Scale bars = 50 μm. (D) Quantification of LC3 positive cells in the ischemic penumbra of the cortex (10f fields/brain). n = 3 per group. ##p < 0.01 versus sham-operated mice, **p < 0.01 versus tMCAO/R mice.
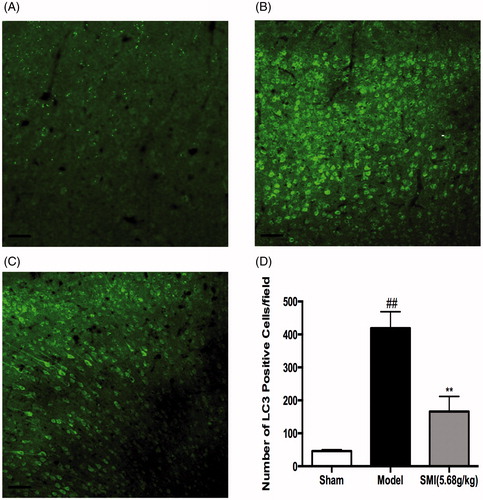
Figure 5. Effects of Shengmai injection (SMI) on the expression of Beclin1 (A) and LC3 (B) in C57 mice subjected to tMCAO/R. The mice were subjected to 1h of ischemia and 24 h of reperfusion (tMCAO/R). SMI (5.68g/kg, ip) was administered after 1 h of ischemia. Representative Western blot analyses showing the levels of Beclin1 and LC3. GAPDH is shown to verify protein loading. The data are the mean ± SD, n = 3. ##p < 0.01 versus sham-operated mice, **p < 0.01 versus tMCAO/R mice.
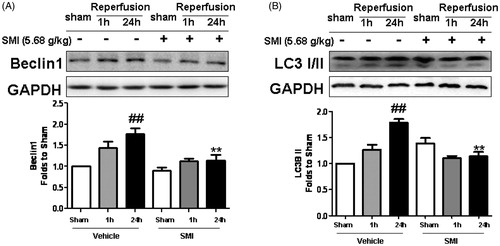
Figure 6. Effects of Shengmai injection (SMI) on the expression and phosphorylation of AMPK, mTOR and JNK in C57 mice subjected to tMCAO/R. The mice were subjected to 1h of ischemia and 24 h of reperfusion (tMCAO/R). SMI (5.68g/kg, ip) was administered after the 1 h of ischemia. Representative Western blot analyses showing phosphorylated and total levels of AMPK, mTOR, JNK. GAPDH is shown to verify protein loading. The data are the mean ± SD, n = 3. #p < 0.05, ##p < 0.01 versus sham-operated mice, *p < 0.05, **p < 0.01 versus tMCAO/R mice.
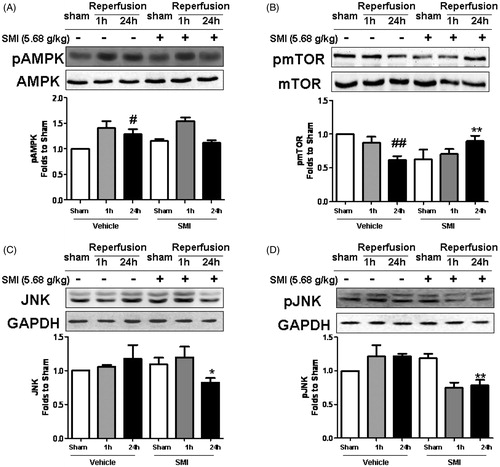
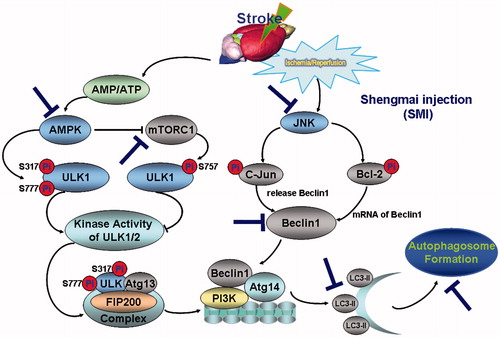
Figure 7. Schematic diagram of the protective effect of Shengmai injection (SMI) against cerebral ischemia/reperfusion in mice via autophagy and related signalling pathways. SMI could remarkably inhibit the phosphorylation of AMPK and down-regulated the phosphorylation of mTOR and JNK after 24 h of reperfusion.SMI could significantly inhibit the autophagy-related proteins including Beclin 1 and LC3, and then decrease the formation of autophagosome so as to suppress the cerebral ischemia/reperfusion in mice.