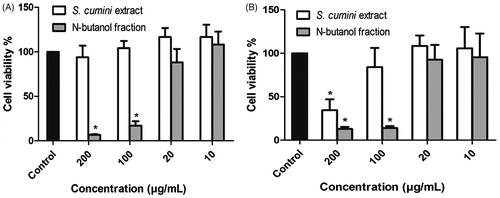
Figures & data
Table 1. Phytochemical markers of the Nb fraction from Sideroxylon obtusifolium hydroalcoholic extract and Syzygium cumini hydroalcoholic extract.
Table 2. Antifungal activity of Sideroxylon obtusifolium extract and its fractions, Syzygium cumini extract, and standard drugs on Candida albicans ATCC 10231 (values are expressed as μg/mL).
Table 3. Effects of the Nb fraction from Sideroxylon obtusifolium extract, and Sc on Candida albicans (ATCC 10231) cell wall biosynthesis (sorbitol assay). Values are expressed as μg/mL.
Table 4. Effect of different concentrations of exogenous ergosterol (100–400 μg/mL) on the antifungal activity of the Nb fraction from Sideroxylon obtusifolium extract; Syzygium cumini extract; and Amphotericin B on Candida albicans ATCC10231.
Figure 1. Effects of the Nb fraction from Sideroxylon obtusifolium extract on biofilm morphology/integrity. SEM photomicrographs (5000x) showing Candida albicans biofilm cells treated with different concentrations of the Nb fraction (A) 62.5 μg/mL (MIC), (B) 250 μg/mL (MFC), (C) 312.5 μg/mL (5xMIC), (D) 625 μg/mL (10xMIC); as well as (E) 0.97 μg/mL nystatin and (F) vehicle. The Nb fraction affected cell structures even at low concentrations (MIC). Nystatin was used as positive control, and the vehicle did not affect the biofilm cells.
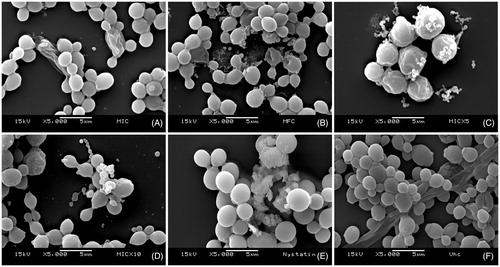
Figure 2. Effects of the Syzygium cumini extract on biofilm morphology/integrity. SEM photomicrographs (5000x) showing Candida albicans biofilm cells treated with different concentrations of Sc: (A) 125 μg/mL (MIC), (B) 500 μg/mL (MFC), (C) 625 μg/mL (5xMIC), (D) 1250 μg/mL (10xMIC); as well as (E) 0.97 μg/mL nystatin and (F) vehicle. It can be noted that Sc affected cell structures at concentrations as low as 125 μg/mL. Nystatin was used as positive control, and the vehicle did not affect the biofilm cells.
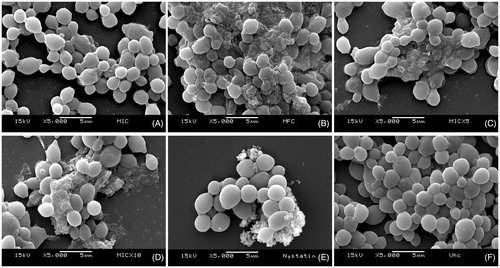
Figure 3. Effects on biofilm viability. This is a representative 2-D confocal imaging of Candida albicans biofilms treated with (A) Nb fraction from S. obtusifolium extract (5xMIC: 312.5 μg/mL); (B) S. cumini hydroalcoholic extract (5xMIC: 625 μg/mL); (C) Nystatin (MIC: 0.97 μg/mL) [positive control]; and (D) Vehicle (negative control). The structures depicted in green (Concanavalin A, Alexa Fluor® 488 Conjugate) represent the yeast cell wall, and those depicted in yellow (FUN® 1 Cell Stain) are nonviable cells, metabolically inactive. The viable cells, in turn, convert the dye FUN-1 to red fluorescent aggregates (63x optical magnitude, 2.85 zoomed-in). Concanavalin A selectively binds to polysaccharides, including alpha-mannopyranosyl and alpha-gluco-pyranosyl residues, and gives a green fluorescence. FUN-1 is a fluorescent dye taken up by yeast cells; in the presence of metabolic viability it is converted from a diffuse yellow cytoplasmic stain to red. It can be noted that both Sc and Nb fraction affected the viability of C. albicans cells when compared to the vehicle.
![Figure 3. Effects on biofilm viability. This is a representative 2-D confocal imaging of Candida albicans biofilms treated with (A) Nb fraction from S. obtusifolium extract (5xMIC: 312.5 μg/mL); (B) S. cumini hydroalcoholic extract (5xMIC: 625 μg/mL); (C) Nystatin (MIC: 0.97 μg/mL) [positive control]; and (D) Vehicle (negative control). The structures depicted in green (Concanavalin A, Alexa Fluor® 488 Conjugate) represent the yeast cell wall, and those depicted in yellow (FUN® 1 Cell Stain) are nonviable cells, metabolically inactive. The viable cells, in turn, convert the dye FUN-1 to red fluorescent aggregates (63x optical magnitude, 2.85 zoomed-in). Concanavalin A selectively binds to polysaccharides, including alpha-mannopyranosyl and alpha-gluco-pyranosyl residues, and gives a green fluorescence. FUN-1 is a fluorescent dye taken up by yeast cells; in the presence of metabolic viability it is converted from a diffuse yellow cytoplasmic stain to red. It can be noted that both Sc and Nb fraction affected the viability of C. albicans cells when compared to the vehicle.](/cms/asset/2a78829e-33c8-4c4f-a9a0-911d0cce9ffe/iphb_a_1155629_f0003_c.jpg)