Figures & data
Figure 1. HIKARI-OLE study design. The diagram depicts the breakdown of HIKARI DB study patients into four groups for the OLE phase of the study. *Regardless of their initial DB phase group assignment, patients who achieved an ACR20 response at Week 12 or 14 as well as at Week 24 were randomized (1:1) to either CZP 200 mg Q2W (Group III, n = 43) or CZP 400 mg Q4W (Group IV, n = 43).
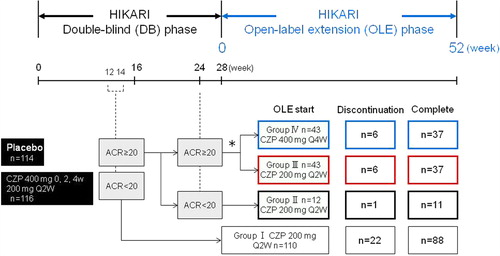
Table 1. Reasons for discontinuation of therapy.
Table 2. Patient demographics and disease status at the HIKARI pre-study baseline (FAS population).
Figure 2. The ACR20/ACR50/ACR70 rates in patients from each treatment group. The percentages of patients in Groups I (n = 110), II (n = 12), III (n = 43), IV (n = 43), and patients in Groups II + III + IV combined (DB completers, n = 98) who achieved an (a) ACR20, (b) ACR50, or (c) ACR70 response were plotted over time for the DB and the OLE phase of the study (FAS population and LOCF imputation). Of note, Week 0 of the OLE phase of Group I (early escape) corresponds to Week 16 of the DB phase. There are no points in the missing section of the graph for Group I.
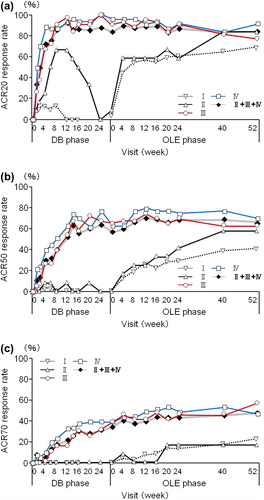
Figure 3. The change of DAS28-ESR and HAQ-DI over HIKARI pre-study baseline in patients from each treatment group. Changes in (a) DAS28-ESR and (b) HAQ-DI from HIKARI pre-study baseline of Groups I (n = 110), II (n = 12), III (n = 43), IV (n = 43), and patients in Groups II + III + IV combined (DB completers, n = 98) were plotted against time for the DB and the OLE phase of the study (FAS population and LOCF imputation). Of note, Week 0 of the OLE phase of Group I (early escape) corresponds to Week 16 of the DB phase. There are no points in the missing section of the graph for Group I.
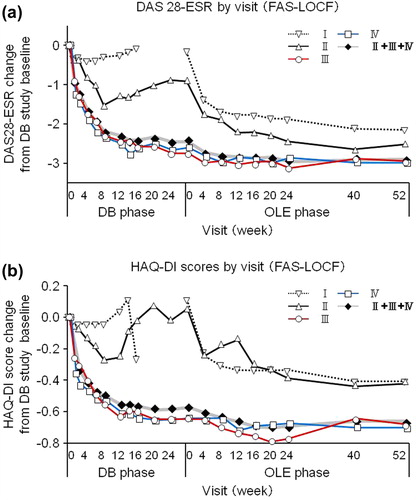
Figure 4. Inhibition of progression of structural damage: cumulative probability plot representing the change from OLE study entry in mTSS at Week 52 (FAS population and linear extrapolation). The graph depicts the cumulative probability of patients displaying a particular change in mTSS from OLE study entry in Groups I (n = 89), II (n = 11), III (n = 38), IV (n = 37), and patients in Groups II + III + IV combined (DB completers, n = 86).
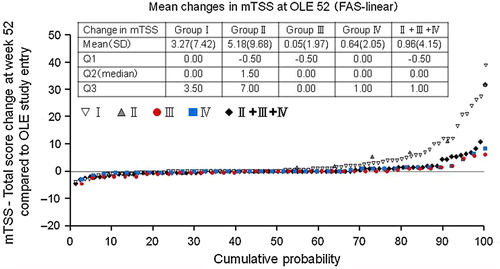
Figure 5. Post-hoc analysis of ACR20/50/80 response rates in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The percentages of patients who achieved an ACR20, ACR50, and ACR70 response of (a) all CZP-DB completers (n = 81), (b) CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and (c) CZP-DB completers treated without additional non-MTX DMARDs (n = 34) were plotted against time for the DB and the OLE phase of the study (LOCF imputation).
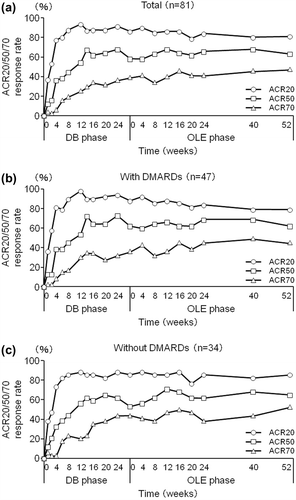
Figure 6. Post-hoc analysis of changes in (a) DAS28-ESR and (b) HAQ-DI scores from HIKARI pre-study baseline in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The DAS28-ESR and HAQ-DI scores of all CZP-DB completers (n = 81), CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and CZP-DB completers treated without additional non-MTX DMARDs (n = 34) were plotted against time for the DB and the OLE phase of the study (LOCF imputation).
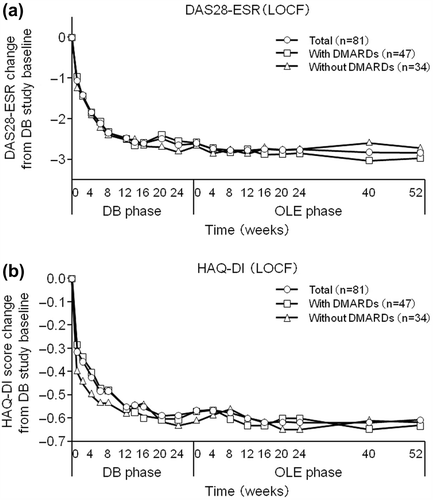
Figure 7. Post-hoc analysis of disease activity states in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The proportions of patients with high (defined as DAS28-ESR > 5.1), moderate (> 3.2 and ≤ 5.1), low (≤ 3.2), or remission (< 2.6) disease activity states among (a) all CZP-DB completers (n = 81), (b) CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and (c) CZP-DB completers treated without additional non-MTX DMARDs (n = 34) at DB Week 0 (DB0), DB Week 24 (DB24), OLE Week 0 (OLE0), OLE Week 24 (OLE24), and OLE Week 52 (OLE52) are shown (LOCF imputation).
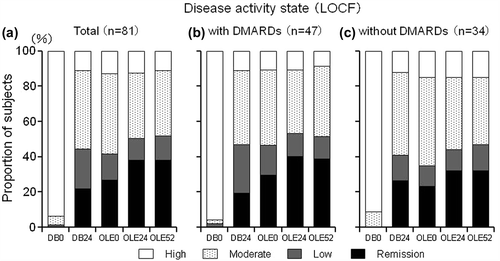
Table 3. Treatment-emergent adverse events.
