Figures & data
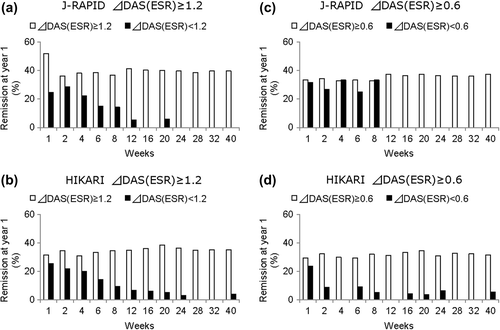
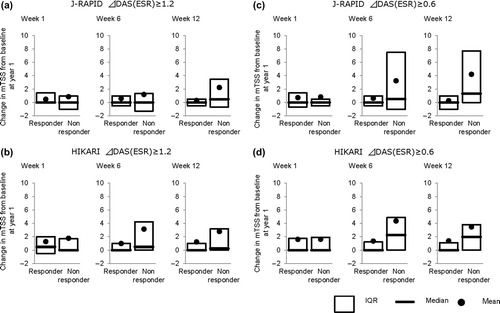
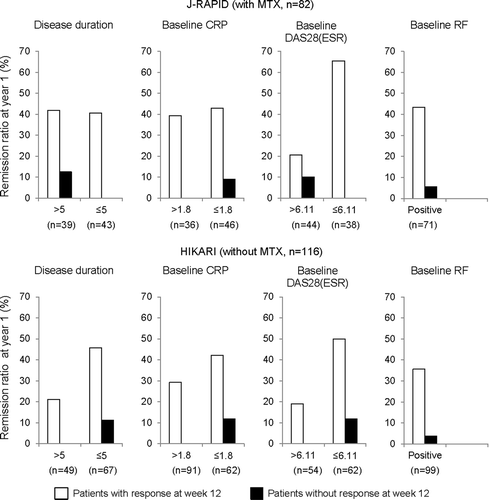
Table 1. Proportions of radiographic non-progression and rapid-radiographic progression after 1 year treatment by response at week 1, 6, and 12 in patients with active rheumatoid arthritis treated with certolizumab pegol with or without MTX.
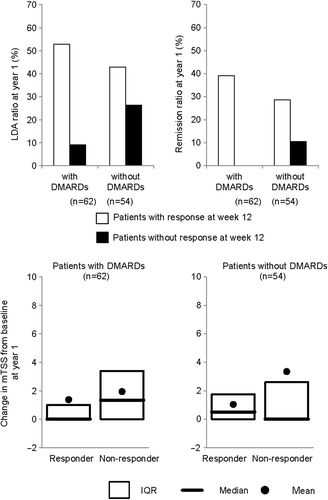
Table 2. Probability of low disease activity or remission after 1 year treatment by response at week 12 in patients with active rheumatoid arthritis treated with certolizumab pegol with or without MTX.
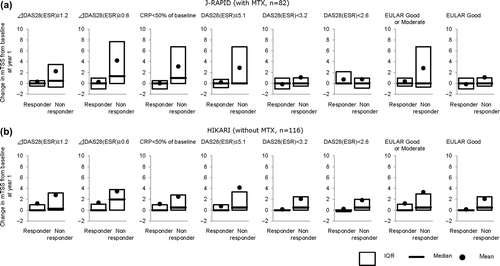
Supplemental material