Figures & data
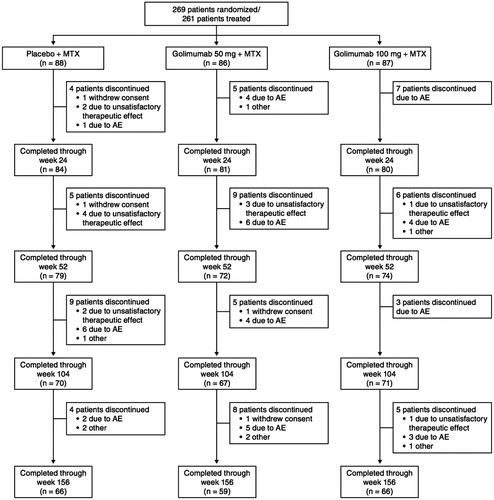
Figure 2. The proportions of patients* with an ACR20 (A), ACR50 (B), and ACR70 (C) response through week 156. *Observed data without imputation. ACR20/50/70, ≥ 20%/50%/70% improvement in American College of Rheumatology criteria; MTX, methotrexate.
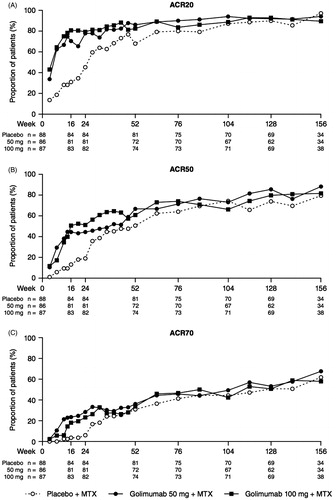
Figure 3. The proportions of patients* with remission as defined by the CDAI (A), SDAI (B), and Boolean (C) criteria through week 156. *Observed data without imputation. CDAI, clinical disease activity index; MTX, methotrexate; SDAI, simplified disease activity index.
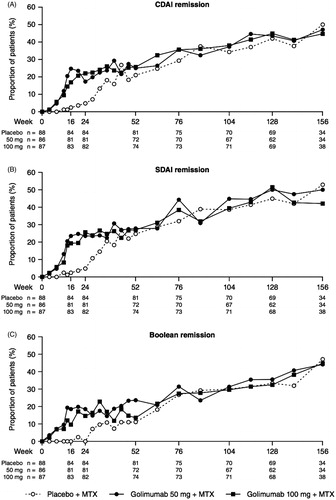
Table 1. Clinical efficacy results at weeks 52, 104, and 156.
Table 2. Radiographic results through 156 weeks.
Table 3. Adverse events through 156 weeks.