Figures & data
Figure 1. Reaction of 2-alkylthio-4-oxo-quinazolineacetonitriles and analogous thieno[3,2-d]pyrimidineacetonitriles with aliphatic thiols to imidazo-fused tricyclic systems by trapping of thioimidates; X = CH=CH or SCitation13.
![Figure 1. Reaction of 2-alkylthio-4-oxo-quinazolineacetonitriles and analogous thieno[3,2-d]pyrimidineacetonitriles with aliphatic thiols to imidazo-fused tricyclic systems by trapping of thioimidates; X = CH=CH or SCitation13.](/cms/asset/77bc4898-f535-4cce-aca0-a60a7f7aca9e/ienz_a_379902_f0001_b.gif)
Scheme 1. Synthesis of compound 3. Reagents and conditions: (a) (NH2)2CS, NaOEt, EtOH, reflux; (b) 1 N NaOH, CH3I, acetone, room temperature; (c) BrCH2CN, (nBu)4NBr, 1 N NaOH, H2O/CH2Cl2.
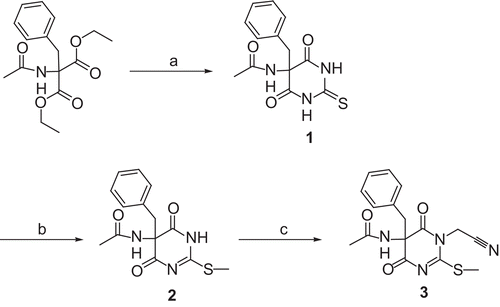
Scheme 2. Synthesis of compound 8. Reagents and conditions: (a) (i) N- methylmorpholine, ClCO2CH2CH(CH3)2, THF; (ii) 25% NH3, −25°C to room temperature; (b) 4 N HCl/dioxane, room temperature; (c) (i) Boc-Phe-OH, N-methylmorpholine, ClCO2CH2CH(CH3)2, THF; (ii) 7 in 1 N NaOH/H2O, −25°C to room temperature; (d) (ClCN)3, DMF, room temperature; (e) CH3I, ethyl acetate, room temperature.
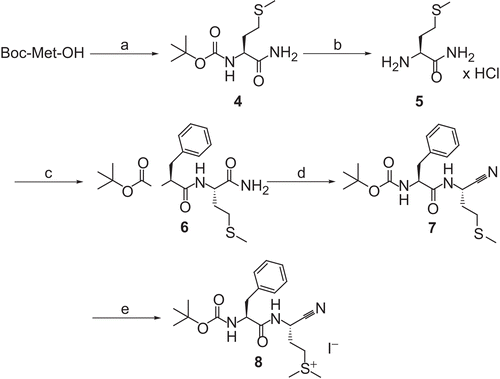
Scheme 3. Synthesis of compound 10. Reagents and conditions: (a) (i) N-methylmorpholine, ClCO2CH2CH(CH3)2, THF; (ii) H-Asn-NH2 × HCl, H2O, 1 N NaOH, −25°C to room temperature; (b) (ClCN)3, DMF, room temperature.
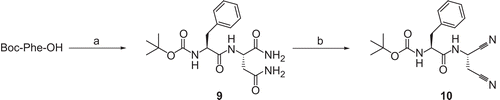
Figure 2. The dihedral angles ϕ and ψ at the peptidic backbone. ϕ is defined by the atoms Ci – 1, Ni, Cαi, and Ci and ψ by Ni, Cαi, Ci, and Ni + 1.
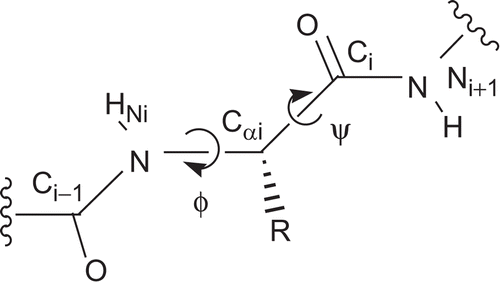
Figure 3. (A) Three-dimensional representation of the l-phenylalanine-derived enantiomer of compound 3. The values for the dihedral angles are ϕ = −80° and ψ = +124°. (B) For comparison, the corresponding open-chain N-acetyl-phenylalanyl-glycine nitrile in the extended, bioactive conformation (ϕ = −139°, ψ = +135°) is shown. Images prepared with PyMOLCitation29.
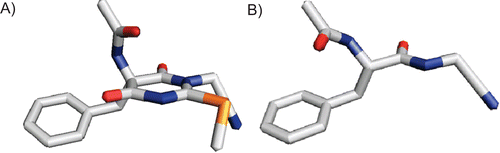
Figure 4. Formation of 2-alkylthio-5-aminopyrroles from succinic dinitrile and thioles (R = CH2COOH) according to Elnagdi et al.Citation32.
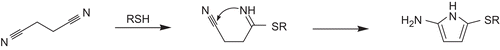
Table 1. Inhibition of cathepsin L, cathepsin S, cathepsin K, and papain by dipeptide nitriles with electrophilic side chains in the P1 position.
Figure 5. (A) Cathepsin S-catalyzed hydrolysis of Z-Val-Val-Arg-NHMec (40 μM) in the presence of increasing concentrations of compound 8 (from top to bottom: 0, 0, 0.3, 0.5, 1.0, 2.0, 3.0, 5.0, 7.0, 10, 20, 100 μM) in 50 mM potassium phosphate pH 6.5, 50 mM NaCl, 2 mM EDTA, 0.01% Triton X-100, 25 μM DTT, 1% DMSO, 37°C. (B) Cathepsin L-catalyzed hydrolysis of Z-Phe-Arg-NHMec (10 μM) in the presence of increasing concentrations of compound 10 (from top to bottom: 0, 0, 1, 2, 3, 4, 5, 7, 10, 15, 50 μM) in 0.1 M sodium phosphate pH 6.0, 0.1 M NaCl, 5 mM EDTA, 0.01% Brij 35, 25 μM DTT, 1% DMSO, 37°C. The reactions were initiated by addition of the enzyme. Fluorescence emission at 440 nm was measured after excitation at 360 nm. Fluorescence units (FU) were corrected for background fluorescence.
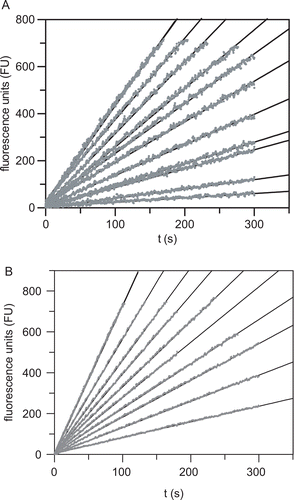
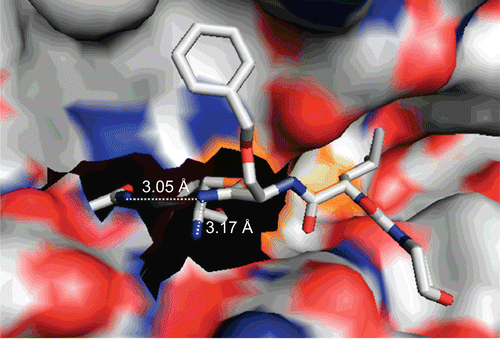
Figure 6. Crystal structure of the complex between cathepsin S and morpholinocarbonyl-leucyl-O-benzylserine nitrile. The thioimidate nitrogen is in hydrogen bond distance to the side chain nitrogen of Gln 19 and the main chain nitrogen of Cys 25. PDB entry 1MS6Citation37, image prepared with PyMOLCitation29.