Figures & data
Figure 1. Chemical structures of cinnamic acid (2), p-coumaric acid (3), ferulic acid (4), sinapic acid (5), and curcumin (6).
Scheme 1. (a) (CH3)2SO4, KOH, 10°C, 1 h, and then reflux 5 h (60%); (b) (Ph3P+=CHCO2C2H5)Br−, KOH, DMSO, 16 h (52%); (c) amines, HOBt, EDCI, TEA, CH2Cl2, room temp., 2 h (67–86%).
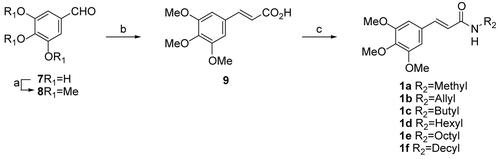
Figure 2. Effects of 3,4,5-trimethoxyphenyl acrylamides on naloxone-induced jumping behavior in morphine-dependent mice. TMCA and 3,4,5-trimethoxyphenyl acrylamides (5 mg/kg, i.p.) and (+)8-OH-DPAT (0.5 mg/kg, i.p.) were injected 30 min before morphine injection (10 mg/kg, i.p.) for 7 days. On the 7th day, naloxone (5 mg/kg, i.p.) was injected 6 h after final drug administration. The number of jumps in a 30 min interval was counted after naloxone injection. *p < 0.05, **p < 0.01 in comparison with morphine only group.
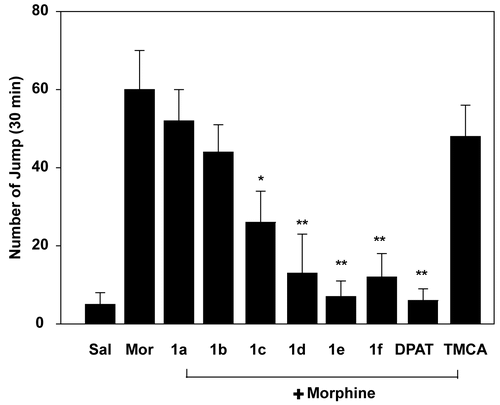
Table 1. Serotonin (5-HT) receptor and transporter binding affinities of 3,4,5-trimethoxyphenyl acrylamides.
