Figures & data
Figure 1. Chemical structures of dimeric bisbenzimidazoles DB(n), bis-Ht (NMe), monomeric bisbenzimidazoles (MB), and Hoeсhst 33258. The numbering of piperazine and benzimidazole hydrogen atoms is shown.
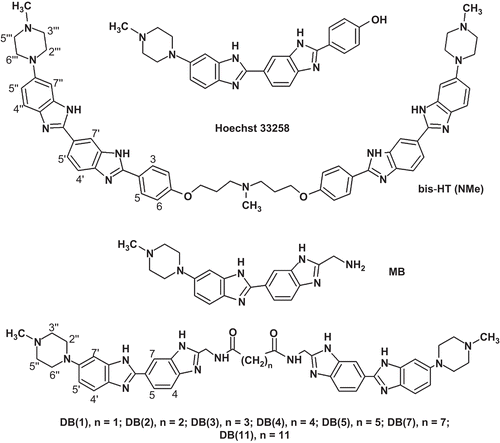
Scheme 1. Synthesis of DB(1–5,7,11). Reagents and conditions: (a) iBuOCOCl, NMM, Boc-Gly-OH; (b) AcOH, 65–70°C, 1 h; (c) AcOH, 120°C, 4 h, 58% (for a–c); (d) HClgas/EtOH, 0–4°C, 1 h, 23°C, 3 days; (e) AcOH/EtOH, 95°C, 1 h, N2, 78% (for d–e); (f) HClconc, 105°C, 20 min, 89%; (g) for DB(1,2): DMF, DIPEA, BOP, HOOC-(CH2)n-COOH (n = 1,2), 0°C, 1 h, 23°C, 1 day, HCl/MeOH, DB(1), 22%, DB(2), 24%; for DB(3–5,7,11): DMF, Et3N, XOOC-(CH2)n-COOX (X = Np; Pfp; Su; n = 3,4,5,7,11), HCl/MeOH, DB(3), 73%, DB(4), 68%, DB(5), 51%, DB(7), 78%, DB(11), 61%. All DB(n) were purified by refluxing in methanol followed by cooling and filtration of the target precipitate. AcOH, acetic acid; Boc-Gly-OH, tert-butyloxycarbonyl-glycine; BOP, (benzotriazole-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; DIPEA, N,N-diisopropylethylamine; DMF, dimethylformamide; Et3N, triethylamine; EtOH, ethanol; iBuOCOCl, isobutyl chloroformate; MeOH, methanol; NMM, N-methylmorpholine; Np, p-nitrophenyl; Pfp, 2,3,4,5,6-pentafluorophenуl; Su, N-oxysuccinimide.
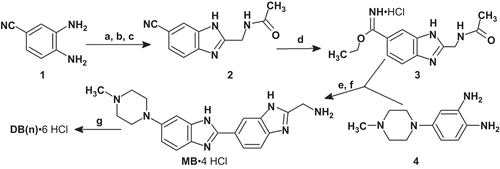
Scheme 2. Synthesis of bis-HT(NMe). Reagents and conditions: (а) Cl(CH2)3N(CH3)(CH2)3Cl, NaH, DMF; (b) HCl, EtOH; (c) AcOH, N2, 120°C, HCl/MeOH, 20%. bis-HT(NMe) was purified by recrystallization from a mixture of MeOH-H2O-HCl.
Table 1. Inhibition of the methylation of duplex A by Dnmt3a-CD in the presence of DB(n).