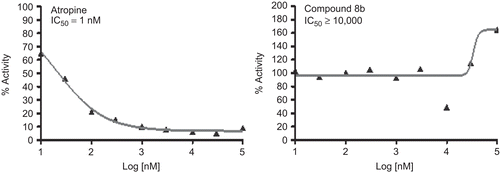
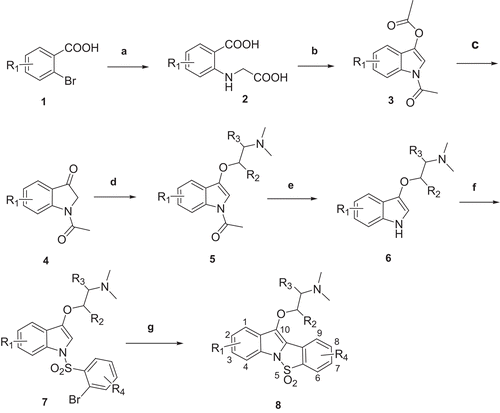
Figures & data
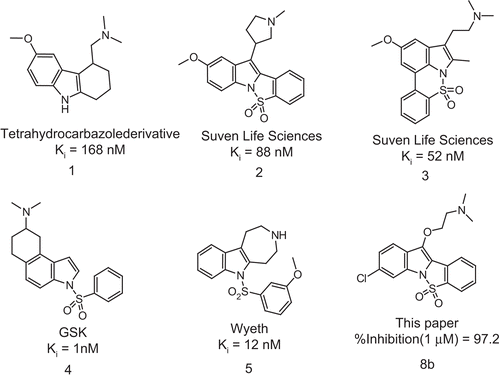
Table 1. 5-HT6R binding affinities.
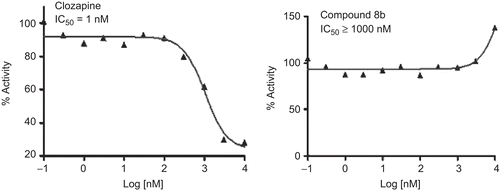
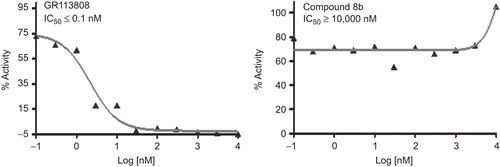
Table 2. Selectivity profile of compounds on other receptors.
Table 3. Human cytochrome P450 inhibitory data and % surrogate metabolism for compounds 8a and 8b.
Table 4. Pharmacokinetic profile of compound 8b in male Wister rats.
Figure 5. Latency to target in Morris water maze task. Data represents mean ± SEM of latency to target recorded as time taken to locate the hidden platform in water. Compound 8b at 10 mg/kg p.o. reversed scopolamine-induced deficit and significant difference from scopolamine-treated group is indicated with asterisk (unpaired t-test; *p < 0.05).
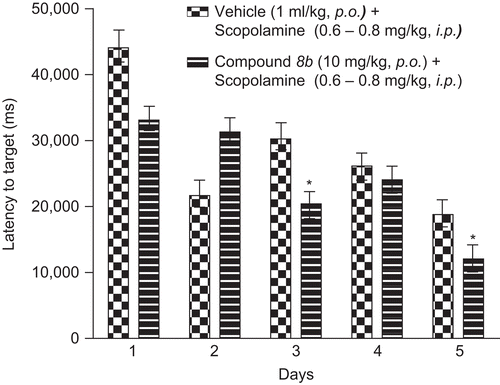
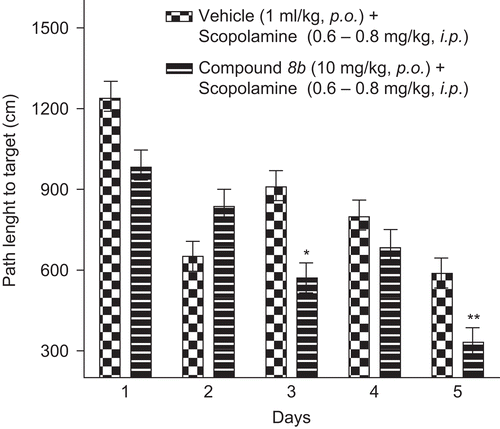
Figure 6. Path length to target in Morris water maze task. Data represents mean ± SEM of path length recorded as path taken to locate the hidden platform in water. Compound 8b at 10 mg/kg p.o. reversed scopolamine-induced deficit and significant difference from scopolamine-treated group is indicated with asterisk (unpaired t-test *p < 0.05; **p < 0.01).