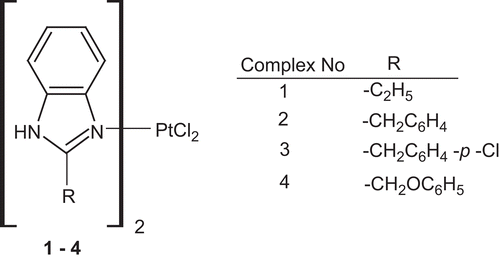
Figures & data
Table 1. Cytotoxicity of benzimidazole–platinum(II) complexes 1–4, cisplatin, and carboplatin on the HeLa, MCF-7, and MDA-MB-231 cell lines.
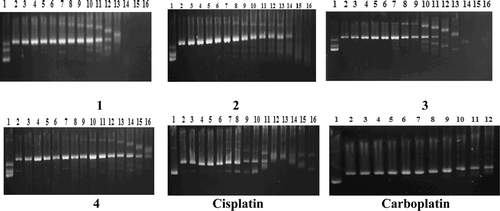
Figure 1. Modification of gel electrophoretic mobility of pBR322 plasmid DNA when incubated with various concentrations of complexes 1–4, cisplatin, and carboplatin. Concentrations (in μM) are as follows. For complexes 1–4 and cisplatin: lines 1 and 16, for carboplatin: lines 1 and 12, untreated pBR322 plasmid DNA; (line 2) 0.019; (line 3) 0.039; (line 4) 0.078; (line 5) 0.156; (line 6) 0.312; (line 7) 0.625; (line 8) 1.25; (line 9) 2.5; (line 10) 5; (line 11) 10; (line 12) 20; (line 13) 40; (line 14) 80; (line 15) 160. For carboplatin: (line 2) 0.312; (line 3) 0.625; (line 4) 1.25; (line 5) 2.5; (line 6) 5; (line 7) 10; (line 8) 20; (line 9) 40; (line 10) 80; (line 11) 160. The top and the bottom bands correspond to Form II (open circular) and Form I (covalently closed circular) plasmids, respectively.
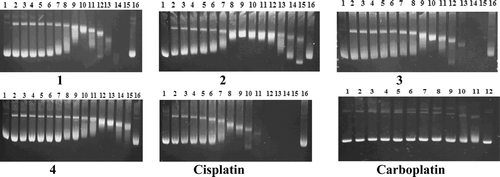
Figure 2. Electrophoretograms for the BamHI-digested mixtures of pBR322 plasmid DNA after their treatment with various concentrations of complexes 1–4, cisplatin, and carboplatin. Concentrations (in μM) are as follows: (lines 1 and 2) untreated pBR322 plasmid DNA and pBR322 DNA linearized by BamHI, respectively. For complexes 1–4 and cisplatin: (line 3) 0.019; (line 4) 0.039; (line 5) 0.078; (line 6) 0.156; (line 7) 0.312; (line 8) 0.625; (line 9) 1.25; (line 10) 2.5; (line 11) 5; (line 12) 10; (line 13) 20; (line 14) 40; (line 15) 80; (line 16) 160. For carboplatin: (line 3) 0.312; (line 4) 0.625; (line 5) 1.25; (line 6) 2.5; (line 7) 5; (line 8) 10; (line 9) 20; (line 10) 40; (line 11) 80; (line 12) 160. The top, bottom, and middle bands indicate Form I (covalently closed circular), Form II (open circular), and Form III (linear) plasmids, respectively.