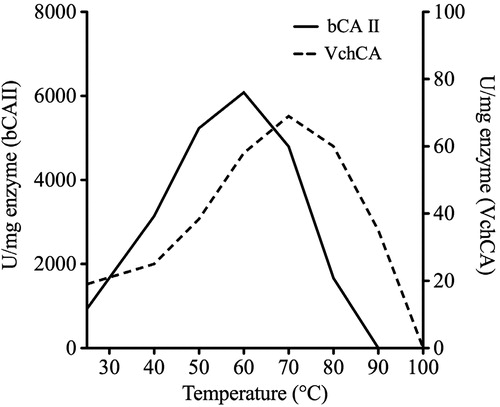
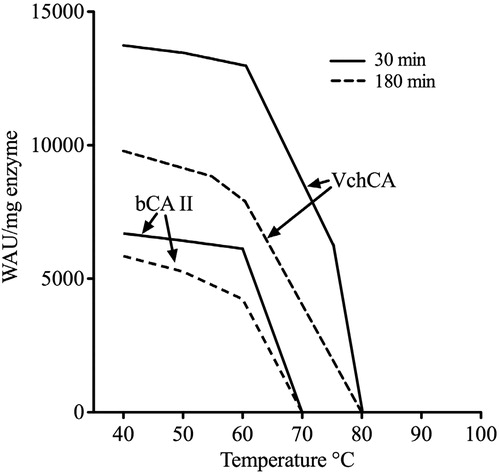
Figures & data
Figure 1. Alignment of the VchCA and bCA II amino acid sequences. The proton shuttle residue (His64), the zinc ligands (His94, 96 and 119) and the gate keeper residues (Glu106 and Thr199) are conserved in the bacterial and mammalian sequences. The hCA I numbering system was used. The bold letters (a, b, c and d) indicate the four loops absent in the bacterial enzyme. The asterisk (*) indicates identity at all aligned positions; the symbol (:) relates to conserved substitutions, while (.) means that semi-conserved substitutions are observed. Multialignment was performed with the program Clustal W, version 2.1. Legend: bCA II, Bos taurus, isoform II (accession number: NP_848667); VchCA, V. cholerae, α-CA (accession number: AFC59768.1).

Figure 2. SDS-PAGE of the recombinant VchCA purified from E. coli. Lane Std, molecular markers, M.W. starting from the top: 250, 150, 100, 75, 50, 37, 25 and 20 k Da; Lane 1, cell extract protein from E. coli before induction with IPTG; Lane 2, cell extract protein after induction with IPTG; Lane 3, supernatant after 45% ammonium sulfate precipitation; and Lane 4, purified VchCA from His-tag affinity column.
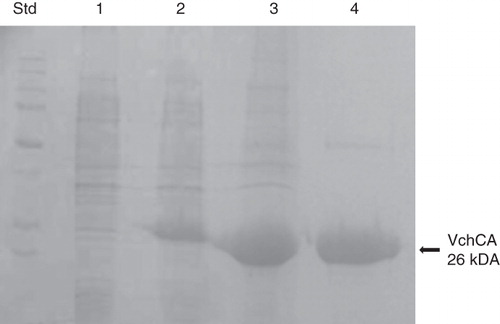
Table 1. Purification of recombinant VchCA produced in E. coli.
Table 2. Comparison of the hydratase and esterase activity for VchCA and bCA II.