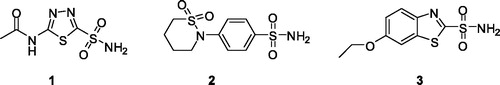
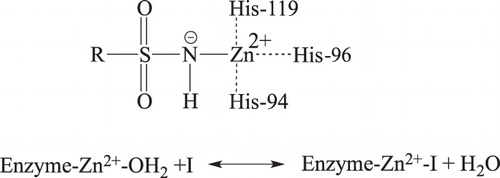
Figures & data
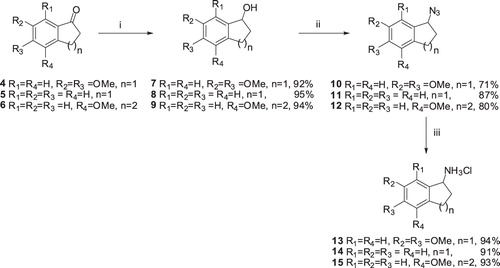
Scheme 2. Synthesis of benzylamine hydrochloride salts: (i) NaBH4, MeOH, 0–25 °C, 3 h; (ii) DPPA/DBU, THF, 0 °C, 2 h, then 25 °C, 12 h, Ar(g); and (iii) H2/Pd-C, CHCl3–MeOH, 25 °C, 20 h.
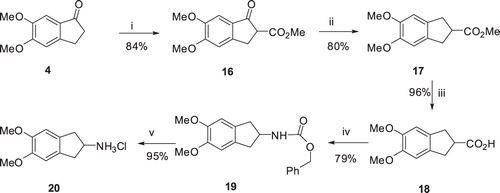
Scheme 3. Synthesis of 2-aminoindane hydrochloride 20: (i) (MeO)2CO/NaH, THF, 66 °C, 12 h; (ii) Et3SiH, TFA, 72 °C, 2.5 h; (iii) a) 4 M NaOH solution, MeOH, 25 °C, 20 h; (b) 37% HCl; (iv) (PhO)2PON3, Et3N, C6H6, reflux, 4 h then PhCH2OH, reflux, 30 h; and (v) H2/Pd-C, CHCl3–MeOH, 25 °C, 20 h.
Scheme 4. Synthesis of sulfonamides: (i) 10% NaOH solution, MeOH, 0–25 °C, 3 h and (ii) MeSO2Cl/NEt3, CH2Cl2, 15 h.
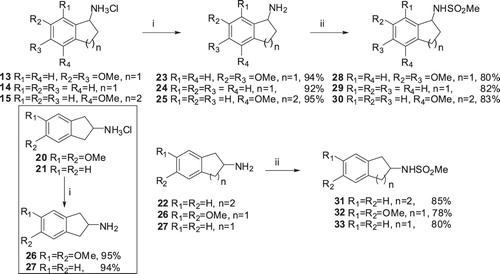
Table 1. Human CA isoenzymes (hCA I and hCA II) inhibition data with some novel sulfonamides 28-33 by an esterase assay with 4-nitrophenylacetate as substrate.