Figures & data
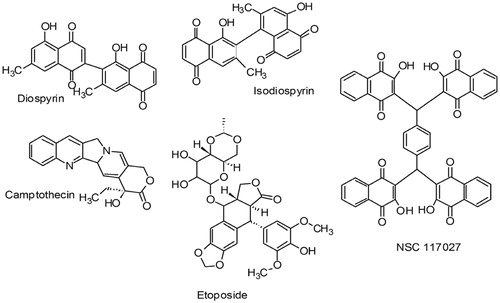
Figure 2. Synthetic scheme of compounds with lawsone (1), different aromatic aldehydes (2), in refluxing condition/H2O/LiCl/12 h and their chemical structures from 3a–e with lawsone dimer.
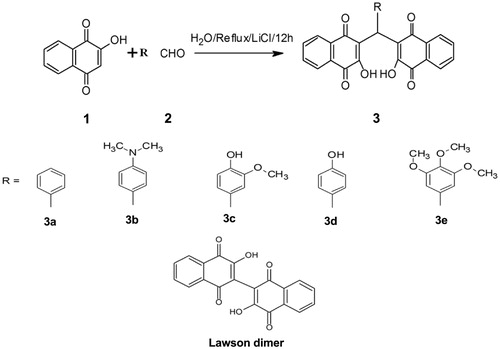
Figure 3. Inhibition of catalytic activity of LdTOP1LS by derivatives bis-naphthoquinone 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4 dione) analogs. (A) Relaxation of super coiled pBS (SK+) DNA with reconstituted LdTOP1LS at a molar ratio of 3:1. Lane 1, 90 fmol of pBS (SK+) DNA; lane 2, same as lane 1, but simultaneously incubated with 30 fmol of LdTOP1LS for 30 min at 37 °C; lane 3, same as lane 2, but in the presence of 2% (v/v) DMSO; lanes 4–5 same as lane 2, but in the presence of 50 and 100 µM of CPT, respectively; lanes 6–17 same as lane 2, but in the presence of 50, 100, 200 µM of compounds 3a, 3b, 3c and 3d, respectively. Positions of super coiled monomer (SM) and relaxed and nicked monomer (RL/NM) are indicated. (B) Preincubation of LdTOP1LS with respective inhibitors followed by the addition of DNA. Lane 1, 90 fmol of pBS (SK+) DNA; lane 2, same as lane 1, but the enzyme was pre-incubated with 2% (v/v) of DMSO; lanes 4–5, same as lane 2, but the enzyme was preincubated with 50 and 100 µM of CPT, respectively; lanes 6–17, same as lane 2, but the enzyme was preincubated with 25, 50, 100 µM of compounds 3a–e, respectively. Reactions were stopped by the addition of SDS to a final concentration of 0.5% and electrophoresed in 1% agarose gel.
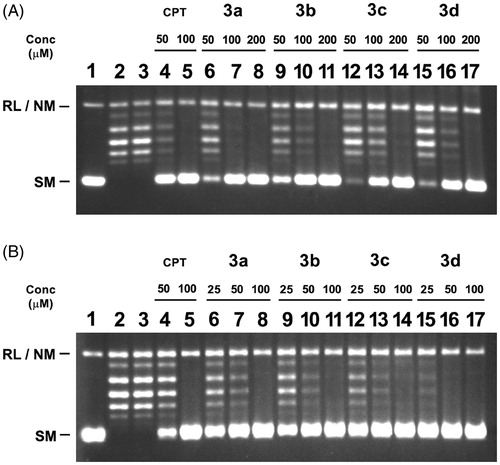
Table 1. Inhibitory concentration of the compounds on LdTOPILS.
Figure 4. Quantitative representation for percentage of promastigotes survival of L. donovani in vitro in the presence of compounds 3a–e and lawsone dimer.
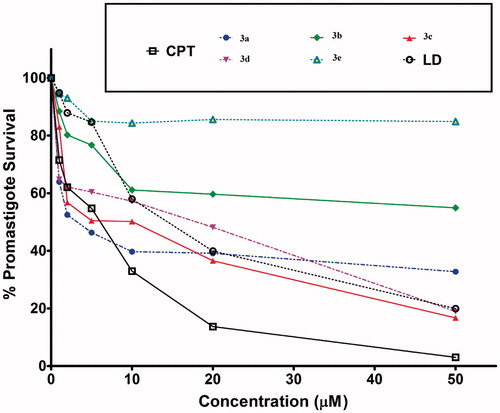
Table 2. EC50 values of the compounds of L. donovani promastigotes.