Figures & data
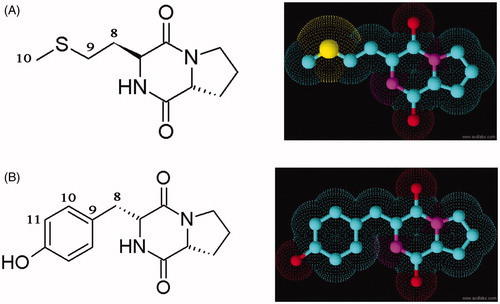
Figure 2. HPLC profile of FDAA derivatives of the acid hydrolysates of DKPs: (A) cyclo(D-Pro-L-Met) and (B) cyclo-(D-Pro-D-Tyr).
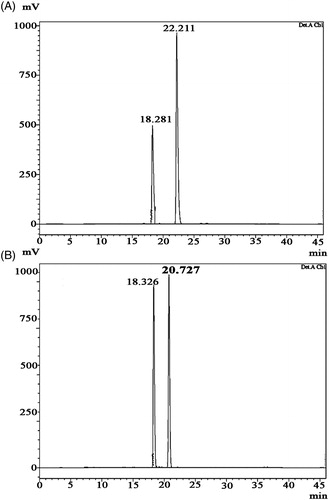
Table 1. HPLC reversed-phase retention times of the standard L and D-amino acid and CDPs derivatives.
Figure 3. HPLC chromatogram of diketopiperazines on a reversed-phase C18 column (LC-20AD). Samples of 15 µl were injected to a column (250 mm × 4.6 mm × 5 mm), eluted with 100% methanol (A) cyclo(D-Pro-L-Met), retention time is 2.711 min. The calculated purity is 98% based on the peak area (B) cyclo-(D-Pro-D-Tyr), retention time is 2.820 min. The calculated purity is 92% based on the peak area.
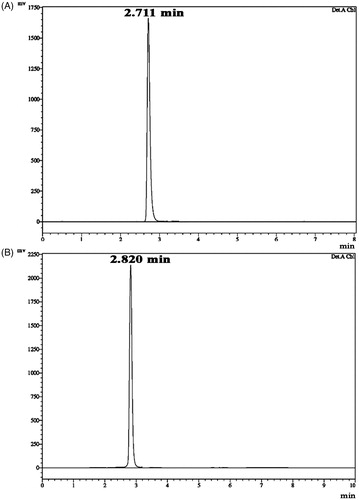
Figure 4. DSC curves of diketopiperazines. Temperatures corresponding to the onset of transition and midpoint of the transition region and enthalpy (ΔH) were recorded by means of the built-in software: (A) cyclo(D-Pro-L-Met) and (B) cyclo-(D-Pro-D-Tyr).
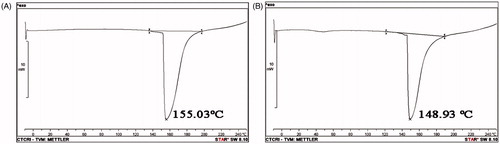
Table 2. NMR data for DKPs in CDCl3 (δ in ppm, J in Hz).
Table 3. MIC of diketopiperazines against test fungi.
Table 4. MIC and MBC of diketopiperazines against test bacteria.
Table 5. Antimicrobial activity of diketopiperazines.