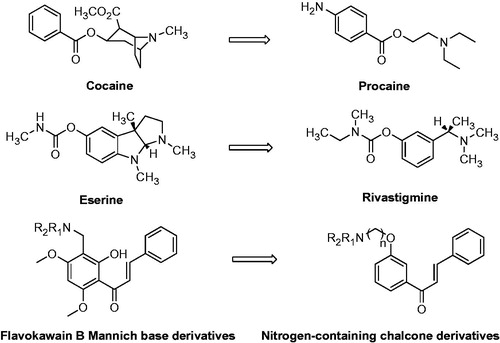
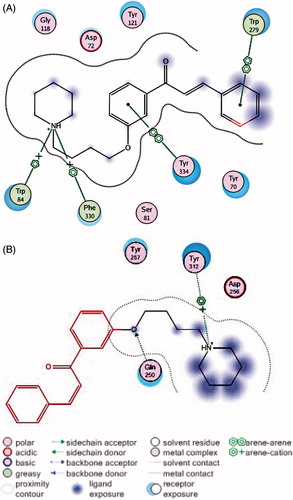
Figures & data
Scheme 1. Reagents and conditions: (a) benzaldehyde, NaOH, EtOH, rt; (b) Br(CH2)nBr, K2CO3, DMF, 80 °C; (c) secondary amine, K2CO3, NaI, acetone, reflux.
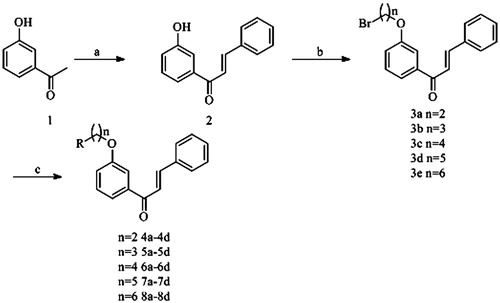
Table 1. Inhibition of AChE and BuChE (IC50 values and selectivity) and log P values of nitrogen-containing chalcone derivatives.
Figure 1. (A) Effects of alkyl chain lengths on anti-AChE activities; (B) effects of alkyl chain lengths on selectivity for AChE.
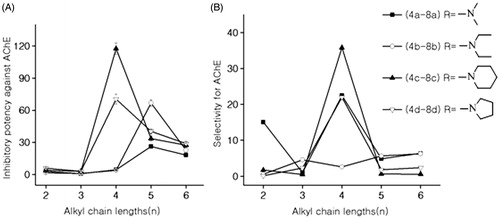
Figure 2. The comparison of the inhibitory activity between the corresponding Flavokawain B derivatives and chalcone derivatives against AChE.
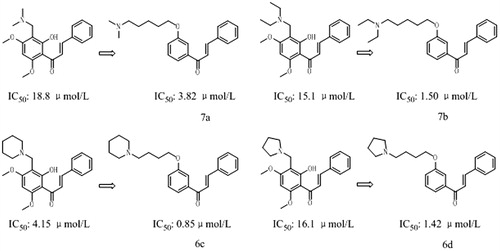
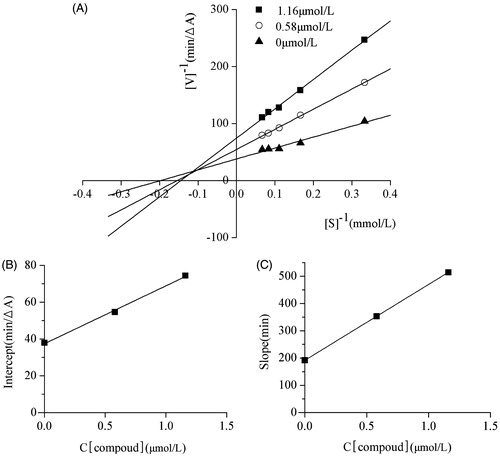
Figure A2. (A) Lineaweaver–Burk plot for the inhibition of AChE by compound 6c; (B) the replots of the intercept versus concentration of compound 6c; (C) the replots of the slope versus concentration of compound 6c.